通过锌克亚胺对取代的吡啶进行元选择性硫代氟烷基化反应
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00533g
引用次数: 0
摘要
锌克亚胺使取代吡啶的区域选择性硫代氟烷基化,光栅获得具有一系列氟化模式的硫代烷基化吡啶。这一战略取得成功的关键是使用了糖精衍生的硫代氟烷基化试剂,这些试剂与tmsl反应后产生亲电性亚砜酰氯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
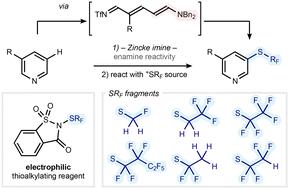
meta-Selective thiofluoroalkylation of substituted pyridines via Zincke imines†
Zincke imines enable the regioselective thiofluoroalkylation of substituted pyridines, providing access to thioalkylated pyridines bearing a range of fluorination patterns. Crucial to the success of this strategy was the use of saccharine-derived thiofluoroalkylating reagents, which, upon reaction with TMSCl, generate electrophilic sulfenyl chlorides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


