Aza-Nazarov环化中的1,2 Wagner-Meerwein变换:Bi(III)催化的依赖底物的高取代吡咯和吲哚的分散合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00168d
引用次数: 0
摘要
Nazarov反应及其变体,如Aza-Nazarov和Iso-Nazarov环化反应是合成包括吡咯和吲哚在内的五元环体系的通用方法。1,2- wagner Meerwein移位与Nazarov和aza -Nazarov样反应以多米诺骨牌顺序结合,分别合成了环戊酮和吲哚衍生物。然而,相同的序列尚未应用于吡咯的合成,可能是由于1-叠氮二烯基阳离子中间体的高反应性。在本报告中,我们提出了用于合成高取代吡咯的Aza-Nazarov/1,2- wagner Meerwein移位多米诺序列的第一个例子。使用铋(III)作为温和的主族金属催化剂,对控制中间体的高反应性至关重要。除了吡咯外,底物还表现出取代基依赖性的产物形成差异,通过异纳扎罗夫环化选择性地给出索引。详细的机理研究揭示了在Lewis和/或“隐式Brǿnsted酸”催化条件下产生的阳离子中间体反应的电环化性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
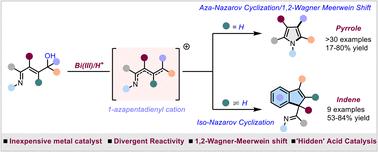
1,2 Wagner–Meerwein shift in aza-Nazarov cyclization: Bi(iii)-catalyzed substrate-dependent divergent synthesis of highly substituted pyrroles and indenes†
The Nazarov reaction and its variants such as aza-Nazarov and iso-Nazarov cyclizations are versatile methods for the synthesis of five-membered ring systems including pyrroles and indenes. The 1,2-Wagner Meerwein shift has been combined in a domino sequence with both Nazarov and aza-Nazarov-like reactions for the synthesis of cyclopentenone and indole derivatives, respectively. However, the same sequence has not been applied for the synthesis of pyrroles, possibly due to the high reactivity of 1-azapentadienyl cation intermediates. In this report, we present the first example of an aza-Nazarov/1,2-Wagner Meerwein shift domino sequence for the synthesis of highly substituted pyrroles. The use of Bi(iii) as a mild main group metal catalyst was found to be crucial to control the high reactivity of the intermediate. The substrate demonstrated substituent-dependent divergence in product formation to selectively give indenes through iso-Nazarov cyclization. Detailed mechanistic investigations reveal the electrocyclization nature of the reaction involving a cationic intermediate generated under Lewis acid and/or ‘hidden Brǿnsted acid’ catalysis conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


