Ni-Al共催化碳磷键活化的机理研究
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00453e
引用次数: 0
摘要
镍铝(Ni-Al)双金属催化在C-P键活化中表现出了显著的效率,但其潜在的机制尚不清楚。关于Ni和Al的协同作用、双催化剂体系的优势以及底物和配体的影响等关键问题对于推进这一策略至关重要。本文采用密度泛函理论(DFT)计算系统地研究了Ni - Al协同催化机理,重点研究了Ni和Al之间的相互作用、AlMe₂Cl的影响、PPh₃的作用以及取代基对反应效率的影响。我们探索了Ni-Al相互作用的两种合作模型,发现不常见的Ni-LA相互作用(模型A)比Ni-LA - l桥接体系(模型B)更有利。这种偏好的产生是因为PPh₃使得苯基迁移或苯基脱氢非常不利,从而将反应导向模型A作为最佳途径。我们的研究结果表明,AlMe₂Cl通过非共价相互作用、电荷重分配、静电稳定和电子密度分布,以及调节HOMO和LUMO能量,有效地降低了激活势垒,在稳定关键过渡态方面起着至关重要的作用。此外,PPh₃通过促进稳定非共价相互作用、通过Ni - p键加强Ni配位和优化电荷转移来提高反应效率。此外,对底物效应的分析表明,空间拥塞和电子稳定都会影响反应效率,更小、更有利电子的取代基有助于电荷转移,降低氧化加成障碍,从而提高实验产率。这些发现为C-P键活化中Ni-Al双金属催化提供了详细的机理理解,并为更有效的催化系统的合理设计提供了指导原则。该研究不仅阐明了Ni和Al的协同作用,而且强调了配体和底物效应对优化反应结果的关键影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
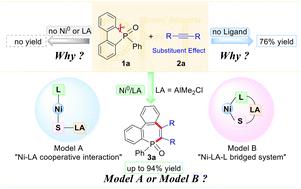
Mechanistic insights into Ni–Al co-catalyzed alkyne carbophosphination enabled by C–P bond activation†
Nickel–aluminum (Ni–Al) bimetallic catalysis has demonstrated remarkable efficiency in C–P bond activation, yet its underlying mechanism remains elusive. Key questions regarding the synergistic roles of Ni and Al, the advantages of the dual-catalyst system, and the effect of the substrate and ligand are critical for advancing this strategy. Here, we employ density functional theory (DFT) calculations to systematically investigate the Ni–Al cooperative catalysis mechanism, focusing on the interplay between Ni and Al, the impact of AlMe2Cl, the role of PPh3, and the effect of substituents on reaction efficiency. We explored two cooperative models for Ni–Al interactions and found that the uncommon Ni–LA interaction (Model A) is more favorable than the Ni–LA–L bridged system (Model B). This preference arises because the use of PPh3 makes phenyl migration or phenyl dehydrogenation highly unfavorable, thereby directing the reaction toward Model A as the optimal pathway. Our results demonstrate that AlMe2Cl plays a crucial role in stabilizing key transition states through non-covalent interactions, charge redistribution, electrostatic stabilization, and electron density distribution, as well as modulating HOMO and LUMO energies, effectively lowering activation barriers. Additionally, PPh3 enhances reaction efficiency by facilitating stabilizing non-covalent interactions, strengthening Ni coordination through a Ni–P bond, and optimizing charge transfer. Furthermore, an analysis of substrate effects reveals that both steric congestion and electronic stabilization influence reaction efficiency, with smaller and more electronically favorable substituents facilitating charge transfer, lowering oxidative addition barriers, and leading to higher experimental yields. These findings provide a detailed mechanistic understanding of Ni–Al bimetallic catalysis in C–P bond activation and offer guiding principles for the rational design of more efficient catalytic systems. The study not only clarifies the synergistic roles of Ni and Al but also highlights the critical influence of ligands and substrate effects in optimizing reaction outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


