通过Breslow中间体和Breslow烯醇酯的碱控制形成从自由基到离子途径切换介离子碳有机催化
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
n -杂环碳(NHC)有机催化技术取得了重大进展。通过Breslow中间体(BIs)和Breslow烯醇化物(BI-s)形成了离子和自由基两种不同的反应途径。选择性生成这些中间体的能力对于优化反应结果至关重要。在本文中,我们证明了介离子碳烯(MICs)可以分别通过使用弱碱和强碱来控制BIs和BI-s的形成。特别令人感兴趣的是醛和烷基卤化物通过离子途径偶联生成酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
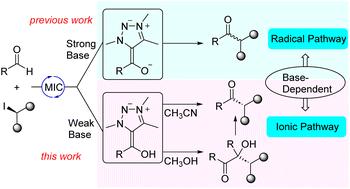
Switching mesoionic carbene-organocatalysis from radical to ionic pathway through base-controlled formation of Breslow intermediates versus Breslow enolates†
N-heterocyclic carbene (NHC) organocatalysis has experienced significant advancements. Two distinct reaction pathways have been developed, ionic and radical, through Breslow intermediates (BIs) and Breslow enolates (BI−s), respectively. The ability to selectively generate these intermediates is crucial for optimizing reaction outcomes. In this paper we show that with mesoionic carbenes (MICs) it is possible to control the formation of BIs versus BI−s, through the use of weak bases and strong bases, respectively. Of particular interest is the coupling of aldehydes and alkyl halides to yield ketones via an ionic pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


