手性磷酸通过动力学分解催化不对称合成具有抗肿瘤活性的C−N轴手性尿嘧啶
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00144g
引用次数: 0
摘要
通过使用手性磷酸催化的不对称酰化法,用非手性氮内酯对 6-NH2 取代的尿嘧啶进行动力学解析,开发出了一种高效的 C-N 轴手性尿嘧啶的不对称合成方法。该方法以高效率和高对映选择性解析了多种 6-NH2 脲嘧啶,选择性系数高达 276。DFT 计算揭示了对映体选择性的来源。大规模反应和手性产物的直接转化凸显了这一方法的实用价值。此外,通过细胞检测还发现了潜在的抗肿瘤药物,说明了这种方法在合成方面的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
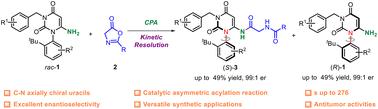
Chiral phosphoric acid catalyzed asymmetric synthesis of C–N axially chiral uracils with antitumor activity through kinetic resolution strategy†
An efficient method for the asymmetric synthesis of C–N axially chiral uracils has been developed by kinetic resolution of 6-NH2-substituted uracils with achiral azlactones, using chiral phosphoric acid-catalyzed asymmetric acylation. A broad range of 6-NH2 uracils were resolved with high efficiency and enantioselectivity, achieving a selectivity factor of up to 276. DFT calculations revealed the origins of the enantioselectivity. Large-scale reaction and straightforward transformations of the chiral products highlighted the practical value of this methodology. Furthermore, potential antitumor agents were identified through cellular assays, illustrating the synthetic utility of this approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


