酸性pH调制硫桥接七元环偶氮吡啶的光开关
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00315f
引用次数: 0
摘要
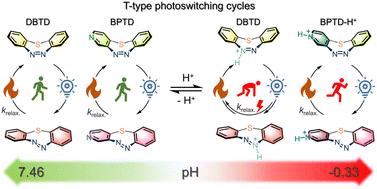
具有双稳态性的偶氮烯分子光开关是光药理学和智能材料制造领域广泛使用的结构调整单元。值得注意的是,中环偶氮苯,尤其是七元二苯并[b,f][1,4,5]硫二氮杂卓(DBTD),具有快速响应的 T 型分子光开关的特点,特别适合将光能转化为环应变能。具有更强双稳态性和溶解性的偶氮杂环烯类化合物的出现大大拓宽了它们的应用范围。在此,我们提出了一类新型的七元环偶氮杂环烯,即苯并[b]吡啶并[f][1,4,5]硫二氮杂卓(BPTD)和二吡啶并[2,3-b:3',2'-f][1,4,5]硫二氮杂卓(DPTD)。BPTD 和 DPTD 中含有吡啶并异戊二烯,因此能在 pH = -0.33 到 7.0 的范围内实现受 pH 调节的 T 型光开关性能,从而与 DBTD 区分开来。重要的是,苯并[b]吡啶并[3,4-f][1,4,5]硫二氮杂卓(3-BPTD)的光开关幅度(E-异构体的光静止分布)略有增强,而且在高酸性环境中具有良好的光稳定性和热稳定性。这些特点使它们有望成为潜在的耐酸光能转换器和耐酸快速反应智能材料的 T 型光电开关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acidic pH-modulated photoswitching of sulfur-bridged seven-membered cyclic azopyridines†
Azoarene molecular photoswitches with bistability are a family of widely employed structure-tuning units for photopharmacology and smart material construction. Notably, medium-ring azobenzenes, especially seven-membered dibenzo[b,f][1,4,5]thiadiazepines (DBTD), are characterized as fast-responsive T-type molecular photoswitches with particular features for light-energy conversion to ring-strain energy. The proliferation of azoheteroarenes with enhanced bistability and solubility has considerably broadened the horizon of their utilization. Herein, we present a novel class of seven-membered cyclic azoheteroarenes, benzo[b]pyrido[f][1,4,5]thiadiazepines (BPTD) and dipyrido[2,3-b:3′,2′-f][1,4,5]thiadiazepine (DPTD). The integration of pyrido-heteroarenes in BPTD and DPTD enables pH-modulated T-type photoswitching performance spanning from pH = −0.33 to 7.0, distinguishing them from DBTD. Importantly, benzo[b]pyrido[3,4-f][1,4,5]thiadiazepine (3-BPTD) exhibits slightly enhanced photoswitching amplitude (photostationary distribution of E isomers) as well as decent photo- and thermal stability in highly acidic environments. These features make them promising T-type photoswitches for potential acid-resistant light-energy converters and acid-endurable fast-responsive smart materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


