用(全氟叔丁基)丙酸高效构造全氟叔丁基炔的一般方案
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00601e
引用次数: 0
摘要
全氟叔丁基(PFtB)脱颖而出,部分原因在于其无与伦比的分析能力,是生物系统中传感和成像不可或缺的功能基团。虽然我们已经完成了sp- 3和sp- 2碳的全氟叔丁基的引入,sp-碳的全氟叔丁基化仍然是一个具有挑战性的任务。在本研究中,我们以全氟叔丁基丙酸(PFtPA)为新试剂,开发了一种多用途的方法来构建结构多样的PFtPA取代炔。PFtPA易于合成,可与多种(芳基、烷基、烯基和炔基)卤化物和三氟酸盐进行Pd/ cu催化脱羧偶联。该合成方案不仅产率高,而且与多种官能团兼容。此外,我们通过开发一种19f标记的探针来证明这种新的合成方案的潜力,该探针能够熟练地区分具有远端手性中心的分析物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
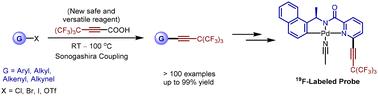
A general protocol for efficient construction of perfluoro-tert-butyl alkynes with (perfluoro-tert-butyl)propiolic acid†
The perfluoro-tert-butyl group (PFtB) stands out, in part, due to its unparalleled analytical capabilities, as an indispensable functional group for sensing and imaging within biological systems. Although previously we have accomplished the introduction of the perfluoro-tert-butyl group into sp3-and sp2-carbons, the perfluoro-tert-butylation of sp-carbon remains a challenging task. In this study, we develop a versatile method to construct structurally diverse PFtB-substituted alkynes, utilizing (perfluoro-tert-butyl)propiolic acid (PFtPA) as a new reagent. PFtPA is easy to synthesize and can undergo Pd/Cu-catalyzed decarboxylative coupling with a wide array of (aryl, alkyl, alkenyl, and alkynyl) halides and triflates. This synthetic protocol is not only high-yielding but also compatible with various functional groups. Furthermore, we demonstrate the potential of this new synthetic protocol by developing a 19F-labeled probe that is proficient in distinguishing analytes with distal chiral centers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


