Oxetane作为现代药物化学工具箱的一部分:3,3-二取代构建块的高级合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-28
DOI:10.1039/d5qo00572h
引用次数: 0
摘要
尽管氧乙烷具有许多潜在的优点,但它们的合成可及性和开环性限制了它们在药物设计中的广泛应用。在这项工作中,我们披露了我们的实验成果和10年的经验,在有机和药物化学家的典型工具箱的反应条件下,我们对氧乙烷核心耐受性进行了全面的研究,这是基于我们原始的,未发表的发展。研究的反应范围包括氧化、还原、烷基化、酰化、亲核取代、C - C/C=C/C≡C键形成、水解和保护基团裂解,并证明了氧乙烷部分在酸性和碱性条件下的稳定性。应用了40多种变换来生成氧乙烷的化学稳定性曲线,这可以指导未来其他合成方法的设计,以合成和纳入氧乙烷。这项工作的另一个目标包括优化合成方案,使单次生产的规模增加到1公斤。我们手中新设计的合成方案允许制备新型3,3-二取代的氧乙烷作为小的构建块(超过100个例子,其中近90%在以前的文献中没有报道)。这些结果有利于氧乙烷作为现代药物化学工具箱的一部分的发展,以及将其纳入药物开发计划。本文章由计算机程序翻译,如有差异,请以英文原文为准。
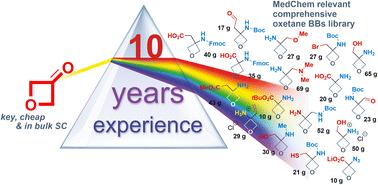
Oxetane as a part of modern medicinal chemistry toolbox: the advanced synthesis of 3,3-disubstituted building blocks†
Despite numerous potential advantages of oxetanes, their restricted synthetic accessibility and propensity to ring-opening hamper their wide application in drug design. In this work, we disclose our experimental achievements and 10 years of experience in oxetane chemistry and provide a comprehensive study on the oxetane core tolerance towards the reaction conditions of the typical toolbox of organic and medicinal chemists, which is based on our original, unpublished developments. The scope of examined reactions includes oxidation, reduction, alkylation, acylation, nucleophilic substitution, C–C/CC/CC bond formation, hydrolysis, and protecting groups cleavage, and demonstrates the stability of the oxetane's moiety towards acidic and basic conditions. Over 40 transformations were applied to generate the oxetane chemical stability profile, which could direct the design of other synthetic approaches for the synthesis and incorporation of oxetanes in the future. The additional aim of the work included the optimization of the synthetic protocols with the possibility of scaling up to 1 kg in a single run. Newly designed synthetic protocols in our hands allowed for the preparation of novel 3,3-disubstituted oxetanes as small building blocks (over 100 examples, almost 90% of which were not reported previously in the literature). These results benefit the development of oxetanes as a part of the toolbox of modern medicinal chemistry, as well as their incorporation into drug development programs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


