相迁移z方案电荷传输使光氧化还原催化利用水作为电子源
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
z -图光催化反应是相当有趣的,因为它们有潜力应用于还原性分子转化为增值化学品,使用水作为电子源。然而,大多数z方案光催化的演示仅限于整体水分解。特别是,在水溶液中,传统的Z-scheme体系基本上不可能将“水不溶性化合物”的还原与水氧化结合起来。在这项工作中,构建了一个具有“相迁移”氧化还原介质的非常规Z-scheme电子传输系统,该系统通过水作为电子/质子源实现水不溶性化合物的光催化转化。在二氯乙烷(DCE)/水双相溶液中,一分子Ir(III)配合物在DCE相中以二茂铁(Fc)为电子供体,作为光氧化还原催化剂催化苄基溴的还原偶联反应。另一方面,负载(Fe,Ru)Ox助催化剂的Bi4TaO8Cl半导体的水分散体以二茂铁(Fc+)作为电子受体光催化水氧化。由于Fc+/Fc的分配系数有显著差异,因此在每个反应中光诱导电子转移产生的Fc+和Fc可以选择性地提取到相反的液相中。自发相迁移使电子在分离反应相中连接还原和氧化反应的有机/水界面上的定向传递成为可能。最后,采用“水作为电子/质子源”的“水不溶性”有机化合物的光催化还原转化,通过逐步进行的z -方案光催化以及相迁移的Fc+/Fc电子传输进行了演示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
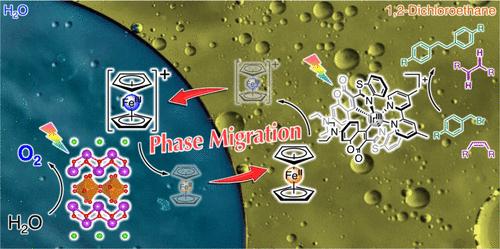
Phase-Migrating Z-Scheme Charge Transportation Enables Photoredox Catalysis Harnessing Water as an Electron Source
Z-schematic photocatalytic reactions are of considerable interest because of their potential for application to reductive molecular conversions to value-added chemicals using water as an electron source. However, most demonstrations of Z-scheme photocatalysis have been limited to overall water splitting. In particular, it has been basically impossible to couple the reduction of “water-insoluble compounds” with water oxidation by conventional Z-scheme systems in aqueous solution. In this work, an unconventional Z-scheme electron transportation system with a “phase-migrating” redox mediator is constructed that enables photocatalytic conversion of water-insoluble compounds by using water as an electron/proton source. In a dichloroethane (DCE)/water biphasic solution, a molecular Ir(III) complex acts as a photoredox catalyst for the reductive coupling of benzyl bromide by using ferrocene (Fc) as an electron donor in the DCE phase. On the other side, an aqueous dispersion of a Bi4TaO8Cl semiconductor loaded with a (Fe,Ru)Ox cocatalyst photocatalyzed water oxidation using ferrocenium (Fc+) as an electron acceptor. Because the partition coefficients of Fc+/Fc are significantly different, the Fc+ and Fc generated by photoinduced electron transfer in each reaction could be selectively extracted to the opposite liquid phase. Spontaneous phase migration enables direction-selective electron transport across the organic/water interface that connects the reduction and oxidation reactions in the separated reaction phase. Eventually, photocatalytic reductive conversion of “water-insoluble” organic compounds using “water as the electron/proton source” was demonstrated through the step-by-step Z-scheme photocatalysis with the phase-migrating Fc+/Fc electron transportation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


