具有多阴离子络合物阴离子的氢化物离子导体
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氢化物离子(H -)导电固体材料近年来在先进的电化学能量存储/转换系统中受到了广泛的关注,包括氢化物离子基电池、电解和燃料电池。然而,氢化物离子的高活性给阴离子系统的多样化带来了挑战,这对设计它们的有效运输至关重要。本研究报道了以聚阴离子硼氢化物(BH4 -)为阴离子的钙钛矿型氢化物离子导体Sr1-yNayLiH3-x-y (BH4)x(0≤x≤1和0≤y≤0.1)。结构表征表明,在立方钙钛矿结构中H -和BH4 -共存的单相氢化物离子导体在低x区稳定。此外,加入H -空位(y的增加)增强了H -和BH4 -的无序性,将氢化物离子的电导率提高了3个数量级。中子粉末衍射分析表明,聚阴离子BH4 -与阳离子(Sr2+和Na+)不对称相互作用,从而促进氢化物离子通过这些相互作用较弱的途径传导。这种不寻常的结构使得氢化物离子在100°C时的电导率超过10-4 S cm-1。目前的研究表明,许多复杂的阴离子有希望成为氢化物离子导体的新型阴离子体系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
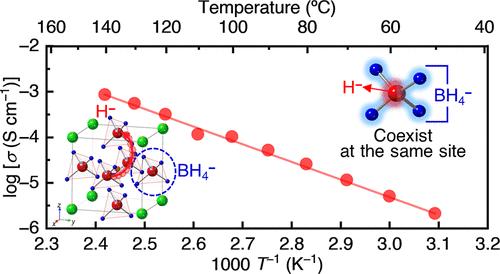
Hydride Ion Conductors with Polyanionic Complex Anions
Hydride ion (H–)-conducting solid-state materials have lately received great attention for advanced electrochemical energy storage/conversion systems, including hydride ion-based batteries, electrolysis, and fuel cells. However, the highly reactive nature of hydride ions has posed challenges in diversifying anion systems, which are crucial for designing their effective transport. This study reports perovskite-type hydride ion conductors, Sr1–yNayLiH3–x–y(BH4)x (0 ≤ x ≤ 1 and 0 ≤ y ≤ 0.1), employing polyanionic borohydride (BH4–) as the anions in the structure. Structural characterization indicates that single-phase hydride ion conductors, in which H– and BH4– coexist in the cubic perovskite structure, are stabilized at the low-x region. In addition, incorporating H– vacancies (an increase in y) enhances the disorder of H– and BH4–, improving hydride ion conductivity by 3 orders of magnitude. Neutron powder diffraction analysis reveals that the polyanionic BH4– interacts asymmetrically with the cations (Sr2+ and Na+), thereby facilitating hydride ion conduction through pathways where these interactions are weaker. This unusual structure allows for a high hydride ion conductivity of over 10–4 S cm–1 at 100 °C. The current study suggests that many complex anions can be promising candidates as novel anion systems for hydride ion conductors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


