光氧化还原催化偶氮苯与烷基硼酸的自由基氢烷基化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
N-烷基-N,N ' -二芳基肼的快速合成一直是一个难题。在这里,我们报道了光氧化还原催化偶氮苯与烷基硼酸的氢烷基化反应,成功地得到了多种N-烷基-N,N ' -二芳基肼。以吖啶盐为光催化剂,在可见光照射下,多种偶氮苯和烷基硼酸在路易斯碱的存在下在常温下顺利反应。机理研究表明,该反应通过生成烷基自由基进行,并由光氧化还原催化促进。本文章由计算机程序翻译,如有差异,请以英文原文为准。
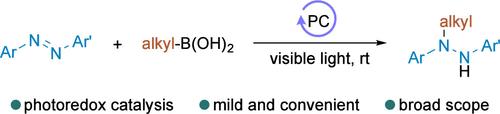
Photoredox-Catalyzed Radical Hydroalkylation of Azobenzenes with Alkylboronic Acids
The facile synthesis of N-alkyl-N,N′-diarylhydrazines has been a long-standing challenge. Here, we report a photoredox-catalyzed hydroalkylation of azobenzenes with alkylboronic acids, which successfully affords diverse N-alkyl-N,N′-diarylhydrazines. With an acridinium salt as the photocatalyst and upon visible light irradiation, a broad range of azobenzenes and alkylboronic acids reacted smoothly in the presence of a Lewis base at ambient temperature. Mechanistic studies revealed that the reaction proceeds via the generation of alkyl radicals and is facilitated by photoredox catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


