pd催化反位位和化学选择性糖基化:弗里达霉素A和喜马拉雅霉素B的全合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这里,我们报告了一种新的合成策略,用于fridamycin型糖苷天然产物。该方法的一个突出特点是pd催化的fridamycin A甲酯与烷氧烯的不对称氢烷氧基化,这使得2,3,6-三脱氧糖苷以高度可控的方式在不同的羟基位置上进行位点和化学选择性的引入。该方法的一个独特优势被证明是完全合成喜马拉雅霉素B和C4 ' -外延衍生物的拟议结构的阿霉素B。本文章由计算机程序翻译,如有差异,请以英文原文为准。
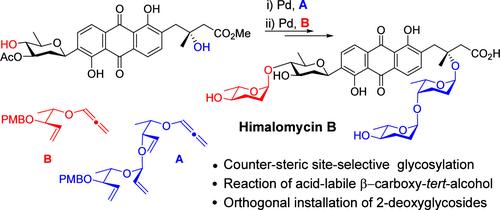
Pd-Catalyzed Counter-Steric Site- and Chemoselective Glycosylation: Total Synthesis of Fridamycin A and Himalomycin B
Here, we report a de novo synthetic strategy toward fridamycin-type glycoside natural products. A salient feature of the method is highlighted by the Pd-catalyzed asymmetric hydroalkoxylation of fridamycin A methyl ester with alkoxyallene, which enables site- and chemoselective introduction of 2,3,6-trideoxyglycosysides to various hydroxyl positions in a highly controlled manner. A unique advantage of this method is demonstrated by the total synthesis of himalomycin B and a C4′-epi derivative of the proposed structure of amicenomycin B.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


