手性诱导的乳酸二聚体对称性偏好的量子隧道指纹图谱
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
乳酸甲酯是一种具有多个官能团的手性分子,对手性方法的实验和理论发展起着举足轻重的作用。利用特定构象的喷射冷却旋转光谱,结合广泛的构象搜索和量子化学计算,我们研究了乳酸甲酯二聚体的手性自识别。实验的指纹图谱,包括甲基转子隧道分裂,允许从大量的低能候选物中确定一个杂手性和两个同手性二元构象。核自旋统计分析和甲基内转子参数揭示了在同手性和异手性环境下不同的核隧穿动力学,并强调了在所观察到的构象中相关的手性驱动的对称偏好。研究结果为手性的量子化学计算基准提供了全面的实验数据,并为使用其他光谱工具在不同频率范围内探索这种原型二聚体铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
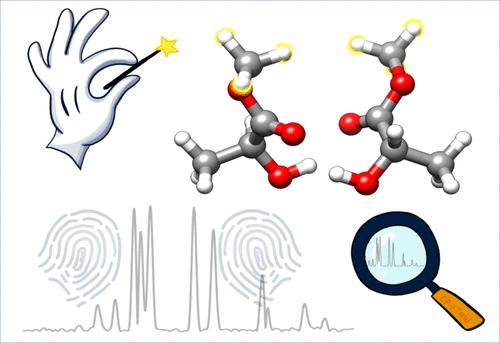
Quantum Tunneling Fingerprints of Chirality-Induced Symmetry Preferences in Methyl Lactate Dimer
Methyl lactate, a chiral molecule with multiple functional groups, has played a pivotal role in advancing experimental and theoretical chiroptical methods. Leveraging conformer-specific jet-cooled rotational spectroscopy in tandem with extensive conformational searches and quantum chemical calculations, we investigated chirality self-recognition in the methyl lactate dimer. The experimental fingerprint-like spectral patterns, including methyl rotor tunneling splittings, allowed the definite identification of one heterochiral and two homochiral binary conformers from a large number of low-energy candidates. Nuclear spin statistics analyses and methyl internal rotor parameters reveal different nuclear tunneling dynamics in the homochiral versus heterochiral environments and highlight the associated chirality-driven symmetry preference in the observed conformers. The results provide comprehensive experimental data for benchmarking quantum chemical calculations of chiral properties and pave the way for the exploration of this prototypical dimer across different frequency ranges using other spectroscopic tools.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


