两步机制的机械化学逆-Diels-Alder-异构体自由基稳定?
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
在有机分子中,杂原子取代是调节化学反应性的一种强有力的分子工程策略。这在聚合物机械化学中是特别有趣的,在聚合物机械化学中,由于机械团的势能表面的力修饰,反应机制可以改变。在这里,我们研究了在蒽-马来酰亚胺(AnM)加合载体的桥接碳旁插入单个杂原子(N, O和S)的影响。之前有人提出,在作用力作用下,反应机制由一致转变为基于均溶键断裂的两步机制,因此,杂原子可能会影响这种转变。事实上,机械化学实验揭示了力诱导的反向diels - alder反应有意义的加速。值得注意的是,硫原子的插入导致了反应性的最大增强,与相同位置的碳原子相比,增加了约2倍。计算研究表明,基于过渡态理论的简单观察并不能解释这种增强的反应性,因此,使用从头算控制的分子动力学,表明键断裂后的自由基寿命与实验结果有很好的相关性。重要的是,实验和计算数据都证实了杂原子插入是增强机械基团反应性的有效和实用的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
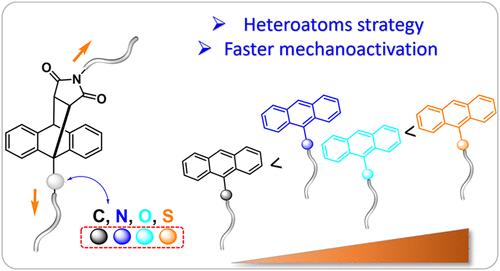
Mechanochemical Retro-Diels–Alder–Heteroatom Radical Stabilization of a Two-Step Mechanism?
In organic molecules, heteroatom substitutions are a powerful molecular engineering strategy for modulating chemical reactivity. This is particularly interesting in polymer mechanochemistry, in which the reaction mechanism can change due to the force modification of the potential energy surface of mechanophores. Here, we investigate the effects of inserting a single heteroatom (N, O, and S) next to the bridging carbon of anthracene–maleimide (AnM) adduct mechanophores. It has been previously proposed that upon application of force, the reaction mechanism changes from concerted to a two-step mechanism based on homolytic bond scission, and therefore, such heteroatoms could affect this conversion. Indeed, the mechanochemistry experiments revealed a meaningful acceleration in force-induced retro-Diels–Alder reaction. Notably, sulfur atom insertion resulted in the highest enhancement in reactivity, with a ca. 2-fold increase compared to a carbon atom at the same position. Computational studies reveal that a simplistic look based on transition state theory does not explain such enhanced reactivity, and therefore, ab initio steered molecular dynamics are used, indicating that the radical lifetime after bond scission correlates well with the experimental results. Importantly, both experimental and computational data confirm that heteroatom insertion is an effective and practical approach to enhancing mechanophore reactivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


