用可见光促进合成吡咯并唑的三组分反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过机械验证的羰基-炔复分解/(3 + 2)-环加成/1,2-芳基迁移顺序,在可见光下由芳基炔、苯醌和5-氨基吡唑组成的三组分级联组装吡咯吡唑。该方案提供吡咯吡唑杂环,收率高达96%,具有优异的官能团耐受性。初步的生物筛选鉴定了所选产品的抗肿瘤活性,突出了该方法的潜在价值。所提出的反应机理得到了对照实验的支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
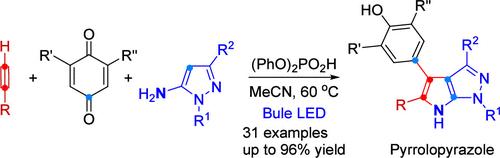
Visible Light-Promoted Three-Component Reaction for the Synthesis of Pyrrolopyrazoles
A visible light-enabled three-component cascade assembles pyrrolopyrazoles from arylalkynes, benzoquinones, and 5-aminopyrazole through a mechanistically validated carbonyl–alkyne metathesis/(3 + 2)-cycloaddition/1,2-aryl migration sequence. This protocol delivers pyrrolopyrazole heterocycles in up to 96% yield with excellent functional group tolerance. Preliminary biological screening identified promising antitumor activity in selected products, highlighting the potential value of this method. The proposed reaction mechanism is supported by control experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


