铜催化β-酰基烯丙基硫醚与烷基溴的还原烷基化反应,以获得具有柔性烷基链的α-支链烯酮
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
用烷基溴化物代替烷基有机金属试剂实现硫醚的脱硫烷基化一直是有机硫化合物脱硫官能化的一个长期挑战。在此,我们报告了在温和的反应条件下,铜催化β-酰基烯丙基硫醚与α-羰基烷基溴的还原叔烷基化反应。该反应具有广泛的底物范围和良好的官能团兼容性,为在α-支链烯酮的烯丙基位置安装多样化的(sp3)-碳季中心提供了直接途径。通过放大实验和产品的后期改性,证明了这一方案在合成方面的进一步应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
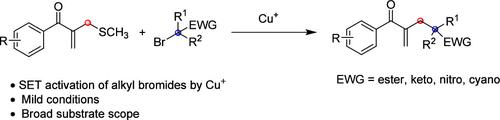
Copper-Catalyzed Reductive Alkylation of β-Acyl Allylic Thioethers with Alkyl Bromides to Access α-Branched Enones with Flexible Alkyl Chains
Replacing alkyl organometallic reagents with alkyl bromides to achieve desulfurative alkylation of thioethers has been a long-standing challenge in desulfurative functionalization of organosulfur compounds. Herein, we report a copper-catalyzed reductive tertiary alkylation of β-acyl allylic sulfides with α-carbonyl alkyl bromides under mild reaction conditions. The reaction accommodates a broad substrate scope with good functional group compatibility, providing a direct route to install diversified (sp3)-carbon quaternary centers at the allylic position of α-branched enones. Further synthetic applications of this protocol have been demonstrated with scale-up experiments and late-stage modification of the products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


