靶向脊髓灰质炎病毒受体激活T细胞,通过IFN-γ-p-STAT1-IRF8轴诱导髓源性抑制细胞分化为促炎巨噬细胞的癌症治疗
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
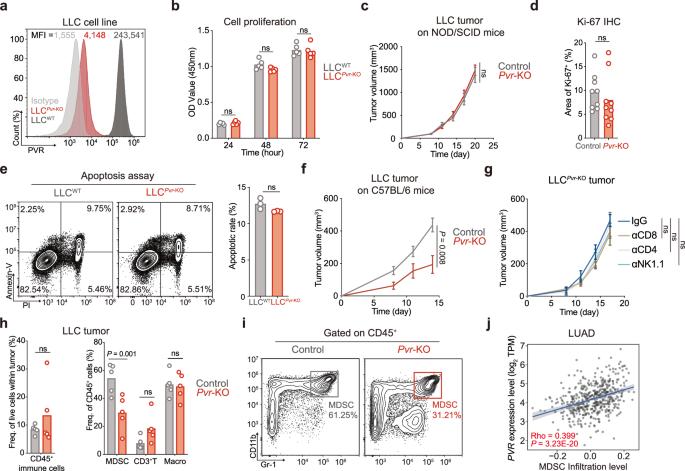
T细胞免疫球蛋白和ITIM结构域(TIGIT)是淋巴细胞上表达的最重要的免疫检查点之一,脊髓灰质炎病毒受体(PVR,又称CD155)是TIGIT最重要的配体,在癌细胞中具有重要功能,影响肿瘤微环境(TME)。虽然已经证实TIGIT阻断可以逆转免疫抑制,但PVR的直接抑制是否会产生类似的结果仍有待充分阐明。本研究研究了PVR在TME中对LLC、CT26和MC38肿瘤模型的作用,发现直接阻断PVR对肿瘤细胞可触发T细胞活化,增强免疫刺激细胞因子IFN-γ的产生,并通过IFN-γ-p- stat1 - irf8轴驱动瘤内骨髓源性抑制细胞(MDSCs)分化为促炎巨噬细胞。此外,本研究发现抗pvr纳米体单药治疗在CT26和MC38肿瘤模型中减少了肿瘤体积。抗pvr纳米体联合抗pd -1抗体在LLC、CT26和MC38肿瘤模型中均有效,毒性可接受。这些发现共同表明,PVR在开发旨在增强抗肿瘤免疫反应的免疫疗法中具有相当大的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Targeting the poliovirus receptor to activate T cells and induce myeloid-derived suppressor cells to differentiate to pro-inflammatory macrophages via the IFN-γ-p-STAT1-IRF8 axis in cancer therapy
T cell immunoglobulin and ITIM domain (TIGIT) is one of the most important immune checkpoints expressed on lymphocytes, and poliovirus receptor (PVR, also CD155) serves as the most crucial ligand for TIGIT, harboring an important function in cancer cells and influencing the tumor microenvironment (TME). While it’s well-established that TIGIT blockade could reverse immunosuppression, the question of whether direct inhibition of PVR yields comparable results remains to be fully elucidated. This study investigated the role of PVR within the TME on the LLC, CT26 and MC38 tumor models and found that direct blockade of PVR on tumor cells could trigger T cell activation, enhance the production of immunostimulatory cytokine IFN-γ, and drive the differentiation of intratumoral myeloid-derived suppressor cells (MDSCs) into pro-inflammatory macrophages through the IFN-γ-p-STAT1-IRF8 axis. Furthermore, this study found that the anti-PVR nanobody monotherapy reduced tumor volume in the CT26 and MC38 tumor models. Combination of anti-PVR nanobody and anti-PD-1 antibody was effective in the LLC, CT26 and MC38 tumor models and had acceptable toxicity. These findings collectively suggest that PVR exhibits considerable promise as a therapeutic target in the development of immunotherapies aimed at augmenting the anti-tumor immune response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


