铱基催化剂对肉桂醛的选择性 CO 加氢及其与多元醇的 C-O 加氢分解的比较†。
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
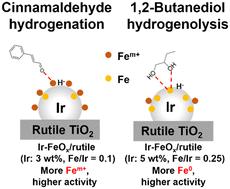
采用低比表面积金红石型TiO2负载ir基双金属催化剂,对肉桂醛选择性加氢制肉桂醇进行了研究。Ir - feox /金红石(Ir: 3wt %, Fe/Ir = 0.1(摩尔基))特别有效。Ir-FeOx /金红石催化剂可用于多种不饱和醛(巴豆醛、糠醛、2-己烯醛和柠檬醛)选择性加氢制不饱和醇(选择性≥95%,产率≥81%)。由于Ir-FeOx/金红石催化剂也被报道能有效地将1,2-二醇氢解为2-单醇,因此我们密切地比较了加氢和C-O氢解的结构-性能关系。与C-O氢解催化剂相比,最佳的加氢催化剂具有较低的Ir负载和较低的Fe/Ir比。此外,催化剂的高温还原降低了肉桂醛加氢的活性,而还原温度对C-O氢解的影响较小。透射电镜(TEM)、CO吸附、XPS和FT-IR表征表明,优化后的催化剂在两种反应中具有相似的结构:fe -合金和fe +修饰合金表面。优化后的催化剂在肉桂醛加氢过程中具有较高的Fe0 / Fem比。从动力学研究(关于H2压力的一级和关于肉桂醛浓度的零级,类似于C-O氢解)来看,氢化物对吸附的肉桂醛的亲核攻击被认为是决定速率的步骤。Ir-Fe合金使吸附的氢具有氢化物性质,而Fem+改性剂作为吸附位点。在肉桂醛加氢过程中,提供肉桂醛的吸附位点更为有效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective CO hydrogenation of cinnamaldehyde over Ir-based catalysts and its comparison with C–O hydrogenolysis of polyols†
Ir-based bimetallic catalysts supported on rutile TiO2 with low surface area were applied to the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol. Ir–FeOx/rutile (Ir: 3 wt%, Fe/Ir = 0.1 (molar basis)) was particularly effective. The Ir–FeOx/rutile catalyst could be applied to the selective hydrogenation of various unsaturated aldehydes (crotonaldehyde, furfural, 2-hexenal and citral) to unsaturated alcohols (≥95% selectivity, ≥81% yield). Since Ir-FeOx/rutile catalysts have also been reported to be effective in C–O hydrogenolysis of 1,2-diols to 2-monoalcohols, the structure-performance relationship was closely compared between hydrogenation and C–O hydrogenolysis. The optimum catalyst for hydrogenation had a lower Ir loading and a lower Fe/Ir ratio than that for C–O hydrogenolysis. In addition, high-temperature reduction of the catalyst decreased the activity in cinnamaldehyde hydrogenation, while the effect of reduction temperature was reported to be small in C–O hydrogenolysis. Characterization using transmission electron microscopy (TEM), CO adsorption, XPS and FT-IR suggested a similar structure for the optimized catalysts in the two reactions: Ir–Fe alloy and Fem+ species modifying the alloy surface. However, a higher Fem+/Fe0 ratio was observed for the optimized catalyst in cinnamaldehyde hydrogenation. From the kinetic studies (first-order with respect to H2 pressure and zero-order with respect to cinnamaldehyde concentration, similar to C–O hydrogenolysis), the nucleophilic attack of the hydride species on the adsorbed cinnamaldehyde was considered the rate-determining step. The Ir–Fe alloy imparted the hydride nature to the adsorbed hydrogen species, and the Fem+ modifier served as the adsorption site. In cinnamaldehyde hydrogenation, supplying adsorption sites for cinnamaldehyde was more effective.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


