氨硼烷与DMSO碱促进n -杂芳烃加氢反应的研究
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了在温和的反应条件下,用氨硼烷和二甲亚砜(DMSO)催化叔丁二氧化钠还原n -杂环芳烃。该方法在不同的n -杂芳烃底物上证明了广泛的官能团相容性。值得注意的是,用氘化的DMSO-d6取代DMSO可以合成c3 -氘化的1,2,3,4-四氢喹啉,具有显著的位置选择性。机理研究表明,质子来源于氨硼烷和二甲基亚砜。该策略为(氘化)n -杂环的合成建立了一种新颖且环保的方法,在操作简单和可持续的反应条件方面具有实际优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
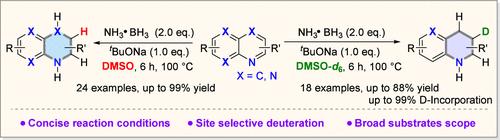
Base Promoted Hydrogenation of N-Heteroarenes with Ammonia Borane and DMSO
Herein, we report a sodium tert-butoxide-promoted reduction of N-heteroarenes using ammonia borane and dimethyl sulfoxide (DMSO) under mild reaction conditions. This method demonstrates broad functional group compatibility across diverse N-heteroarene substrates. Notably, substituting DMSO with deuterated DMSO-d6 enables the synthesis of C3-deuterated 1,2,3,4-tetrahydroquinolines with remarkable positional selectivity. Mechanistic investigations indicate that the protons are derived from both ammonia borane and DMSO. This strategy establishes a novel and environmentally benign approach for the synthesis of (deuterated) N-heterocycles, offering practical advantages in terms of operational simplicity and sustainable reaction conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


