铑催化环1,3-二羰基衍生碘酰化物与环丙醇的偶联
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了环1,3-二羰基与环丙醇通过环碘化策略的模块化α-单烷基化反应。该方法一般,无碱,操作简单,适用于各种医学上重要的(杂)环1,3-二羰基。各种各样的环丙醇,很容易从原料化学品中制备,可以作为模块化和补充烷基化剂。重要的是,产物中新形成的羰基为许多合成应用提供了一个通用的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
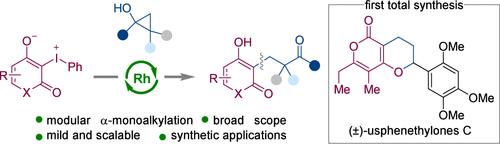
Rh-Catalyzed Coupling of Cyclic 1,3-Dicarbonyl-Derived Iodonium Ylides with Cyclopropanols
Herein, we report a modular α-monoalkylation of cyclic 1,3-dicarbonyls with cyclopropyl alcohols through a cyclic iodonium ylide strategy. This approach is general, base-free, operationally simple, and suitable for various medically important (hetero)cyclic 1,3-dicarbonyls. A wide range of cyclopropyl alcohols, easily prepared from feedstock chemicals, can serve as modular and complement alkylating agents. Importantly, the newly formed carbonyl groups in the resulting products provide a versatile platform for numerous synthetic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


