支架适应性P450酶平衡底物混杂性和催化特异性在芸苔烯生物合成中的应用
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们研究了荆芥烯生物合成途径中两种 P450 酶 AbnK 和 AbnG 的功能可塑性。通过体内异源表达、喂养试验和体外反应,我们发现这两种酶以不同的方式对 5-8-5 和 5-9-5 类萜支架进行了区域和立体选择性转化,并得到了计算模拟的支持。AbnK 还弥补了荆芥烯 A 生物合成过程中的一个缺失步骤。我们的研究结果表明了受控的杂交性是如何支撑结构多样性的,为扩大化学合成的策略提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
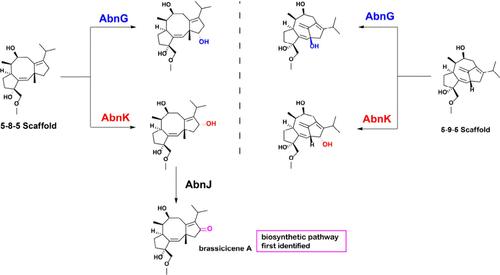
Scaffold-Adaptive P450 Enzymes Balance Substrate Promiscuity with Catalytic Specificity in Brassicicene Biosynthesis
We investigated the functional plasticity of two P450 enzymes, AbnK and AbnG, in the brassicicene biosynthetic pathway. Through in vivo heterologous expression, feeding assays, and in vitro reactions, we show that these enzymes regio- and stereoselectively transform both 5–8–5 and 5–9–5 terpenoid scaffolds in different ways, supported by computational simulations. AbnK also bridges a missing step in brassicicene A biosynthesis. Our findings demonstrate how controlled promiscuity underpins structural diversity, informing strategies to expand chemoenzymatic synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


