对映选择性催化脲醛酸合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-15
DOI:10.1039/d5qo00378d
引用次数: 0
摘要
5,5-二碳取代的邻苯二甲酸酯是许多药物的关键骨架,但这种支架的对映选择性从头合成的通用方法尚不明确。另一方面,UHS是一种有效的制备氢妥英的方法,但其对映选择性变体尚不清楚。基于去对称和动力学分解策略,我们在此公开了第一个不对称催化UHS的例子,提供了具有高立体选择性的合成挑战性硫代氢酰脲。利用易接近的2-氨基丙二酸酯和外消旋氨基酯在手性酸存在下分别与异硫氰酸酯反应。所得产物可以很容易地功能化,并作为各种药物的关键支架。实验研究和DFT计算表明,酯氨解步骤中意想不到的动态动力学分辨率是对映体控制的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
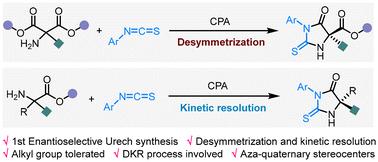
Enantioselective catalytic Urech hydantoin synthesis†
5,5-Dicarbon-substituted hydantoins are the key skeletons of numerous drugs, but a general method for the enantioselective de novo synthesis of such scaffolds is elusive. On the other hand, Urech hydantoin synthesis (UHS) represents an efficient approach for hydantoin preparation, but its enantioselective variant remains unknown. Based on desymmetrization and kinetic resolution strategies, we disclose herein the first example of asymmetric catalytic UHS, providing synthetically challenging thiohydantoins with high stereoselectivities. Readily accessible 2-amino malonic esters and racemic amino esters were employed to react with isothiocyanates in the presence of chiral acids, respectively. The resulting products can be facilely functionalized and serve as pivotal scaffolds in various drugs. Experimental studies and DFT calculations suggest that an unexpected dynamic kinetic resolution in the ester ammonolysis step is responsible for the enantiocontrol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


