can介导的2-脱氧糖的合成:获得手性2-脱氧3-双吲哚- c -糖苷
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
2-脱氧糖和双吲哚在天然产物中普遍存在,在药物化学和化学生物学中起着至关重要的作用。在此,我们报道了一种新颖、快速、高效的技术,利用硝酸铈铵(CAN)在1-2小时内从各种受保护的甘油合成2-脱氧糖。此外,我们进一步探索了在室温下使用三氯化铟(InCl3)催化量将2-脱氧糖转化为手性纯的2-脱氧-3-双吲哚- c -糖苷分子支架的新工艺。该方法强调继承立体多样性和广泛的底物范围,可以合成各种新的2-脱氧3-双吲哚- c -糖苷类似物,为进一步探索其在药物化学和化学生物学中的应用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
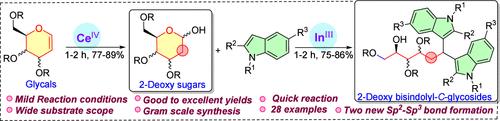
CAN-Mediated Synthesis of 2-Deoxy Sugars: Access to Chiral 2-Deoxy 3-Bisindolyl-C-glycosides
The 2-deoxy sugars and bisindole moieties are ubiquitously found in natural products and play a crucial role in medicinal chemistry and chemical biology. Herein, we report a novel, quick, and highly effective technique that utilizes ceric ammonium nitrate (CAN) to synthesize 2-deoxy sugars from various protected glycals within 1–2 h. Additionally, we further explore the new process of transforming 2-deoxy sugars into chirally pure 2-deoxy-3-bisindolyl-C-glycosides molecular scaffolds using a catalytic amount of indium trichloride (InCl3) in good yields at room temperature. This method emphasizes inherited stereo diversity and a wide substrate scope, which enables the synthesis of various new 2-deoxy 3-bisindolyl-C-glycoside analogues, paving the way for further exploration of their application in medicinal chemistry and chemical biology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


