基于fermi的Sabatier反应动力学模型:Sabatier原理及超越
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二氧化碳甲烷化反应(也称为Sabatier反应)是将废碳转化为更大价值的重要途径。在这项研究中,通过考虑基于电子理论的弱和强化学吸附概念,提出了通过CO途径进行的Sabatier反应的动力学模型。基于Sabatier反应的单路机理,用经典的Sabatier火山形状证明了反应速率与催化剂费米能级位置相对于试剂(CO2)和产物(CH4)的反键态的关系。该模型用于解释镍基和钌基催化体系反应速率的实验结果,并了解动态催化建立的周转频率增强的来源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
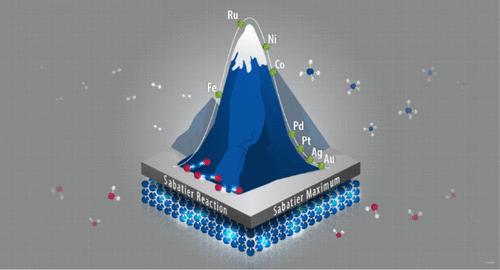
Fermi-Based Kinetic Model for the Sabatier Reaction: Sabatier Principle and Beyond It
The CO2 methanation reaction (also known as the Sabatier reaction) is an important route to convert waste carbon into greater value. In this study, kinetic modeling of the Sabatier reaction proceeding via the CO route has been presented by considering weak and strong chemisorption concepts rooted in electronic theory. Based on the single-route mechanism of the Sabatier reaction, the dependence of the reaction rate on the position of the catalyst Fermi level with respect to the antibonding states of the reagent (CO2) and the product (CH4) is demonstrated with the classic Sabatier volcano shape. The model was applied to explain experimental results of reaction rates for Ni- and Ru-based catalytic systems and to understand the origin of the enhancement of turnover frequencies established for dynamic catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


