动脉粥样硬化中的甲酰肽受体 1 及其拮抗剂 T0080
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
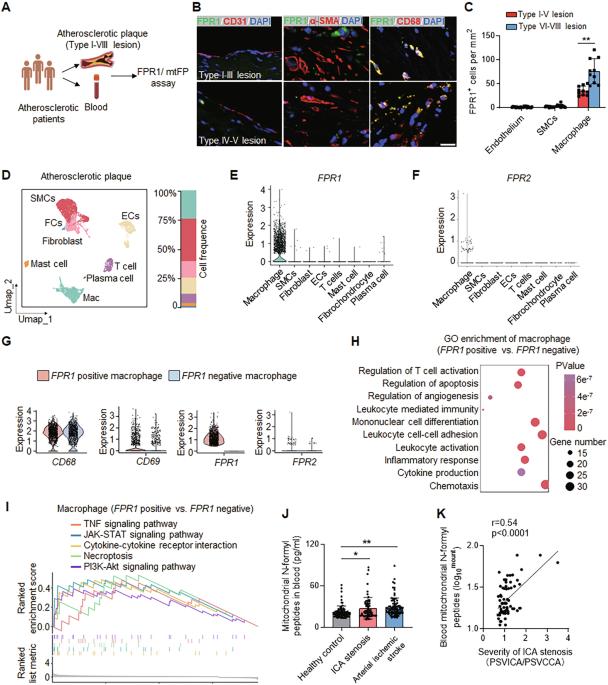
由巨噬细胞驱动的局部炎症和动脉损伤是动脉粥样硬化的关键致病过程。然而,人们对这些过程的调控机制仍然知之甚少。在这项研究中,我们证明了甲酰肽受体 1(FPR1)激动剂(一种线粒体 N-甲酰肽)在动脉粥样硬化患者血液中升高,并与颈动脉狭窄相关。在动脉粥样硬化病变中发现了表达 FPR1 的巨噬细胞。在载脂蛋白 E(ApoE)-/-小鼠中,巨噬细胞中 FPR1 的条件性缺失可减少斑块形成、局部炎症和主动脉粥样硬化。FPR1 能激活巨噬细胞中的蛋白激酶 C(PKC),促进活性氧(ROS)、肿瘤坏死因子α(TNF-α)和白细胞介素-1β(IL-1β)的产生,从而加速内皮细胞和平滑肌细胞的凋亡。为了抑制 FPR1 的生物活性,我们开发了一种拮抗剂 T0080。治疗性服用 T0080 可减轻载脂蛋白E-/-小鼠的动脉粥样硬化进展。我们的研究结果突显了 FPR1 在巨噬细胞介导的动脉粥样硬化斑块形成中的关键作用,并支持进一步研究 T0080 介导的 FPR1 抑制作为动脉粥样硬化的一种潜在治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Formyl peptide receptor 1 and its antagonist T0080 in atherosclerosis
Focal inflammation and arterial damage driven by macrophages are key pathogenic processes in atherosclerosis. However, the mechanisms that regulate these processes remain poorly understood. In this study, we demonstrate that formyl peptide receptor 1 (FPR1) agonist, a mitochondrial N-formyl peptide, is elevated in the blood of patients with atherosclerosis and correlates with carotid stenosis. Macrophages expressing FPR1 were found in atherosclerotic lesions. Conditional deletion of Fpr1 in macrophages reduced plaque formation, local inflammation, and aortic atherosclerosis in apolipoprotein E (ApoE)−/− mice. FPR1 activates protein kinase C (PKC) in macrophages, promoting the production of reactive oxygen species (ROS), tumor necrosis factor alpha (TNF-α) and interleukin-1beta (IL-1β), which accelerates the apoptosis of endothelial cells and smooth muscle cells. To inhibit FPR1 bioactivity, we developed an antagonist, T0080. Therapeutic administration of T0080 attenuates atherosclerotic progression in ApoE−/− mice. Our findings highlight the pivotal role of FPR1 in macrophage-mediated atherosclerotic plaque formation and support further investigation of T0080-mediated FPR1 inhibition as a potential treatment for atherosclerosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


