HIF-1α诱导的LncRNA-MANCR在缺氧条件下通过靶向miRNA-494/SIRT1信号轴驱动胰腺癌恶性进展的作用
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
这项研究揭示了缺氧诱导的lncRNA-MANCR(MANCR)的潜在生物学作用和基本机制,MANCR在胰腺癌(PC)中显著上调。这项研究揭示了缺氧诱导的MANCR的潜在生物学作用和基本机制,MANCR在胰腺癌中显著升高。微阵列检测证实了MANCR在PC患者和慢性胰腺炎(CP)患者组织中的表达,它与sirtuin-1(SIRT1)mRNA水平呈正相关。研究人员采用染色质免疫沉淀和荧光素酶测定法来评估缺氧诱导因子-1α(HIF-1α)/MANCR/miRNA-494/SIRT1通路中的结合情况。此外,MANCR的表达与PC患者的临床预后之间的关系也得到了证实。缺氧条件下,MANCR在PC细胞中明显上调,这与PC患者的不良预后密切相关。消耗MANCR可抑制PC细胞的体外增殖、迁移和侵袭以及PC异种移植瘤的体内生长。我们进一步证实,MANCR定位于细胞质,并与直接靶向SIRT1的miR-494竞争性结合。从机制上讲,SIRT1的过表达通过去乙酰化提高了HIF-1α蛋白的稳定性,从而增强了HIF-1α的组装。此外,在缺氧环境下,MANCR 会受到 HIF-1α 的转录调控。这种调控归因于HIF-1α与MANCR启动子序列中的缺氧反应元件结合。数据显示 MANCR 和 HIF-1α 之间可能存在反馈作用,这可能有利于缺氧诱导的致癌和 PC 肿瘤的发生,从而提供了一个合适的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
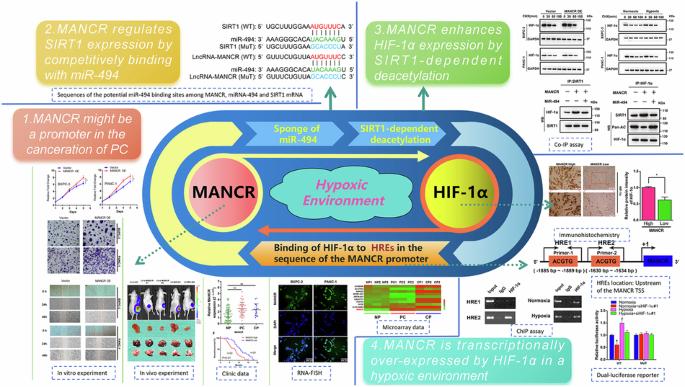
The role of LncRNA-MANCR induced by HIF-1α drive the malignant progression of pancreatic cancer by targeting miRNA-494/SIRT1 signaling axis under hypoxic conditions
This study revealed the prospective biological role and fundamental mechanisms of hypoxia-induced lncRNA-MANCR (MANCR), which is notably upregulated in pancreatic cancer (PC). This work uncovered the potential biological function and underlying mechanisms of hypoxia-induced MANCR, which is significantly elevated in PC. Microarray assays confirmed MANCR expression in the tissues of patients with PC and patients with chronic pancreatitis (CP), which positively correlated with sirtuin-1 (SIRT1) mRNA levels. Chromatin immunoprecipitation and luciferase assays were employed to gauge binding within the hypoxia-inducible factor-1α (HIF-1α)/MANCR/miRNA-494/SIRT1 pathway. Additionally, the association between MANCR expression and the clinical outcomes of patients with PC was confirmed. MANCR is significantly upregulated in PC cells under hypoxic conditions, which is closely linked to poor prognosis in patients with PC. Depletion of MANCR repressed in vitro proliferation, migration, and invasion of PC cells and in vivo growth of PC xenograft tumours. We further demonstrated that MANCR is localised in the cytoplasm and competitively binds miR-494, which directly targets SIRT1. Mechanically, the overexpression of SIRT1 improved the stability of the HIF-1α protein through deacetylation, leading to enhanced HIF-1α assembly. Moreover, MANCR underwent transcriptional regulation by HIF-1α in a hypoxic setting. This modulation was ascribed to HIF-1α binding to hypoxia response elements present in the MANCR promoter sequence. Data revealed the potential possibility of feedback between MANCR and HIF-1α, which may be conducive to hypoxia-induced oncogenicity and PC tumorigenesis, thereby providing a suitable therapeutic target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


