nhc催化Umpolung不对称合成青霉素呋喃酮A
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
第一次不对称全合成青霉素醌A是通过nhc催化的umpolung策略完成的,共八个步骤。该合成的主要特征包括Al-Salen催化的不对称氰基硅化反应,以安装gregatin A的叔醇,以及NHC催化的steter - aldol级联反应。苄基醛片段的umpolung策略促进了与聚甲素a的会聚形式[4+2]环,最终导致青霉素醌a的形成本文章由计算机程序翻译,如有差异,请以英文原文为准。
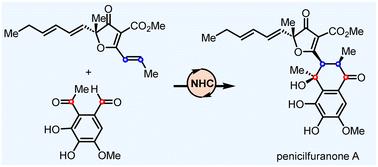
Asymmetric total synthesis of penicilfuranone A through an NHC-catalyzed umpolung strategy†
The first asymmetric total synthesis of penicifuranone A was accomplished in eight steps through an NHC-catalyzed umpolung strategy. Key features of the synthesis include an Al-Salen catalyzed asymmetric cyanosilylation to install the tertiary alcohol of gregatin A, and an NHC catalyzed Stetter–Aldol cascade reaction. The umpolung strategy of the benzyl aldehyde fragment facilitated a convergent formal [4 + 2] annulation with gregatin A, ultimately leading to the formation of penicifuranone A.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


