氧化应激调节的GCLC去琥珀酰化保护人类癌细胞免于铁下垂
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
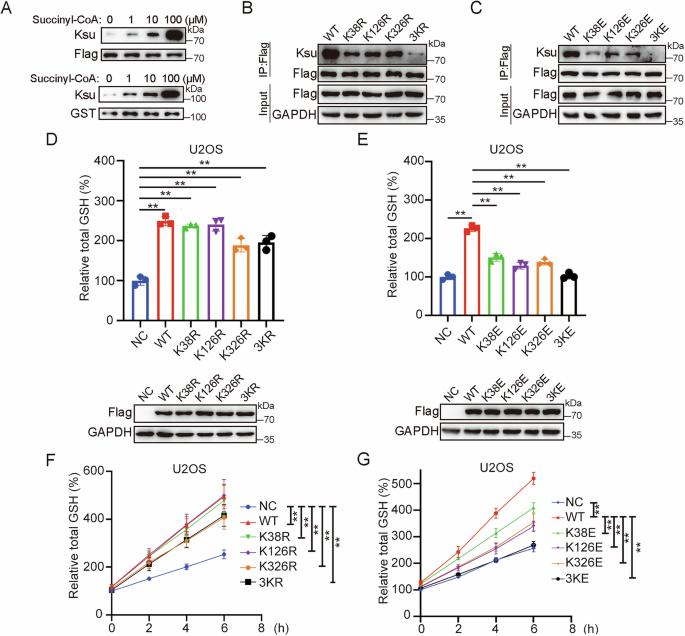
肿瘤细胞进化出强大的抗氧化能力,以抵消肿瘤微环境中异常高水平的活性氧(ROS)。合成抗氧化谷胱甘肽(GSH)的谷氨酸-半胱氨酸连接酶催化亚基(GCLC)是维持肿瘤细胞氧化还原稳态的关键酶,但其活性是否受到琥珀酰化等翻译后修饰的调控尚不清楚。在这里,我们通过体外和体内实验证明了琥珀酰化修饰在GCLC上的存在。nad依赖性去乙酰化酶Sirtuin-2 (SIRT2)作为去琥珀酰化酶,在K38、K126和K326位点催化GCLC去琥珀酰化。具体来说,GCLC直接与SIRT2相互作用,在ROS处理后SIRT2可以显著增强。这种增强的关联导致GCLC去琥珀酰化和活化,从而促进GSH合成并使癌细胞抵抗铁凋亡诱导。SIRT2的缺失降低了总GSH水平,同时增加了细胞对铁凋亡的易感性,这主要可以通过引入野生型GCLC而不是其3K-E突变体来挽救。我们进一步证实组蛋白乙酰转移酶P300在GCLC中起琥珀基转移酶的作用,ROS处理后其相关性显著降低。因此,sirt2调控的GCLC琥珀酰化是癌细胞维持氧化还原平衡以应对氧化应激诱导的铁凋亡的重要信号轴。本文章由计算机程序翻译,如有差异,请以英文原文为准。

GCLC desuccinylation regulated by oxidative stress protects human cancer cells from ferroptosis
Tumor cells evolve strong antioxidant capacities to counteract the abnormal high level of reactive oxygen species (ROS) in the tumor microenvironment. Glutamate-cysteine ligase catalyzing subunit (GCLC) for synthesis of antioxidant glutathione (GSH) represents the key enzyme to maintain redox homeostasis of tumor cells, however, whether its activity is regulated by posttranslational modifications, such as succinylation, remains to be clarified. Here, we demonstrate the existence of succinylation modification on GCLC by in vitro and in vivo assays. NAD-dependent deacetylase Sirtuin-2 (SIRT2) serves as the desuccinylase and catalyzes GCLC desuccinylation at sites of K38, K126, and K326. Specifically, GCLC directly interacts with SIRT2, which can be substantially enhanced upon ROS treatment. This strengthened association results in GCLC desuccinylation and activation, consequently promoting GSH synthesis and rendering cancer cells resistant to ferroptosis induction. Depletion of SIRT2 decreases total GSH level and meanwhile increases the cellular susceptibility to ferroptosis, which can mostly be rescued by introducing wild-type GCLC, but not its 3K-E mutant. We further demonstrated that histone acetyltransferase P300 serves as the succinyltransferase of GCLC, and their association is remarkably decreased after ROS treatment. Thus, SIRT2-regulated GCLC succinylation represents an essential signaling axis for cancer cells to maintain their redox balance in coping with oxidative stress-induced ferroptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


