卵泡蛋白耗竭通过MiT/TFE激活导致肝细胞损伤和胆管癌
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
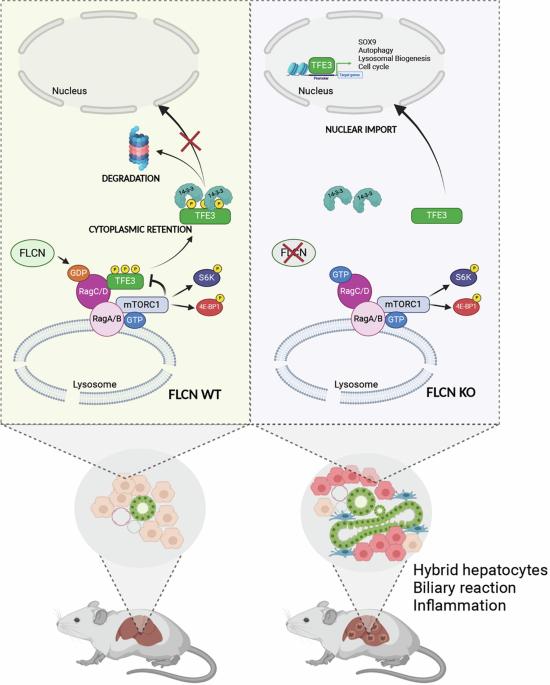
肿瘤抑制基因滤泡蛋白(FLCN)的突变是导致BHD综合征的原因,BHD综合征是一种罕见的遗传性疾病,易使受影响的个体患皮肤肿瘤、肺囊肿和肾肿瘤。FLCN调节关键的细胞通路,包括TFEB、TFE3和mTORC1,它们对维持细胞稳态至关重要。FLCN的缺失会导致mTORC1的过度激活以及TFEB和TFE3的组成性激活,从而促进肿瘤的发生。虽然先前的研究表明Flcn肝脏特异性条件敲除(FlcnLiKO)小鼠在高脂肪饮食暴露下免受肝纤维化和肝损伤的影响,但Flcn缺失在肝癌发生中的潜在作用仍未被探索。在这里,我们证明了FLCN在小鼠肝脏中的缺失导致与胆管癌(CCA)特征的炎症和纤维化相关的癌症。这种表型出现在90周龄以上的小鼠中,雄性占优势。此外,FlcnLiKO小鼠更容易发生二乙基亚硝胺(DEN)-或3,5-二氧羰基-1,4-二氢碰撞碱(DDC)-诱导的具有异质组织学特征的肝脏肿瘤。值得注意的是,FlcnLiKO小鼠肝脏中TFE3而不是TFEB的缺失完全恢复了癌症表型并使mTORC1信号正常化,这表明在FLCN缺失的情况下,TFE3是肝癌和mTORC1过度活跃的主要驱动因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Folliculin depletion results in liver cell damage and cholangiocarcinoma through MiT/TFE activation
Mutations in the tumor suppressor gene Folliculin (FLCN) are responsible for Birt-Hogg-Dube’ (BHD) syndrome, a rare inherited condition that predisposes affected individuals to skin tumors, pulmonary cysts, and kidney tumors. FLCN regulates key cellular pathways, including TFEB, TFE3, and mTORC1, which are critical for maintaining cell homeostasis. Loss of FLCN leads to both hyperactivation of mTORC1 and constitutive activation of TFEB and TFE3, contributing to tumorigenesis. While previous studies showed that Flcn liver-specific conditional knockout (FlcnLiKO) mice are protected from developing liver fibrosis and damage upon high-fat diet exposure, the potential role of FLCN loss in liver carcinogenesis remained unexplored. Here, we demonstrate that hepatic loss of FLCN in mice results in cancer associated with inflammation and fibrosis with features of cholangiocarcinoma (CCA). This phenotype emerges in mice over 90-week-old, with a male predominance. Moreover, FlcnLiKO mice are more prone to develop diethylnitrosamine (DEN)- or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)- induced liver tumors with heterogenous histological features. Notably, depletion of TFE3, but not TFEB, in the liver of FlcnLiKO mice fully rescues the cancer phenotype and normalized mTORC1 signaling, highlighting TFE3 as the primary driver of liver cancer and mTORC1 hyperactivity in the absence of FLCN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


