铜催化苯乙烯与烷烃和酰胺的对映选择性三组分羧酰胺化反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从现成和低成本的化学原料中高效组装有价值的手性分子仍然是当今合成化学中最具挑战性的任务之一。自由基介导的烯烃三组分碳胺化为解决这一挑战提供了一种有吸引力的策略。然而,大多数现有的报告都集中在外消旋的例子上,并且很大程度上局限于活化的烯烃、预活化的烷基化试剂或足够活跃的亲核试剂。在此,我们报道了苯乙烯与未活化的烷烃和弱亲核酰胺的高度对映选择性三组分碳酰胺化反应。通过使用手性阳离子铜催化剂实现对映选择性控制。该方法可合成多种具有优异对映选择性的光学活性酰胺。机理研究表明,该反应是通过烷烃的氢原子转移和烯烃的自由基加成进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
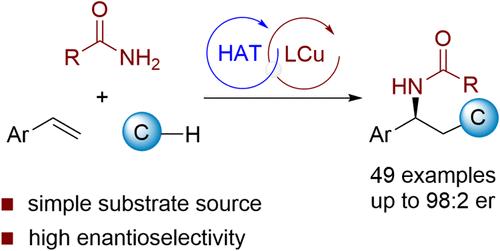
Copper-Catalyzed Enantioselective Three-Component Carboamidation of Styrenes with Alkanes and Amides
Efficient assembly of valuable chiral molecules from readily available and low-cost chemical feedstocks remains one of the most challenging tasks in synthetic chemistry today. Radical-mediated three-component carboamination of alkenes offers an attractive strategy for addressing this challenge. However, most existing reports focus on racemic examples and are largely limited to activated alkenes, preactivated alkylation reagents, or sufficiently active nucleophiles. Herein, we report a highly enantioselective three-component carboamidation of styrenes with unactivated alkanes and weakly nucleophilic amides. Enantioselective control is achieved by using chiral cationic copper catalysts. This method enables the synthesis of a variety of optically active amides with excellent enantioselectivity. Mechanistic studies reveal that the reaction proceeds via hydrogen atom transfer from the alkane followed by radical addition to the olefin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


