抗利尿激素注射液中相关物质的反相高效液相色谱(RP-HPLC)定量方法的建立与验证:抗利尿激素制剂样品在不同稀释液中抗利尿激素(RLD)的表征与对比分析
IF 1.1
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究建立并验证了一种灵敏、准确、可靠、简便的抗利尿激素(VPS)注射液中相关物质的反相高效液相色谱(RP-HPLC)定量方法。开发阶段优先优化波长、流动相组成和色谱柱选择,以提高VPS注射液的分离度和灵敏度。考虑峰对称性、分辨率和保留时间,建立了VPS进样的色谱条件。采用YMC PACK ODS AM (100 ×4.6) mm, 3µm。以0.113 M NaH2PO4制备流动相A。流动相B为乙腈与水的50:50 (v/v)比,泵送流速为1.0 mL/min。将VPS注射液暴露于热、光解、酸、碱和过氧化物降解条件下,并用现有方法对这些条件进行了分析。该方法按照国际协调理事会指南(ICH)制定的指南进行验证。线性研究表明,VPS与相关物质的相关系数大于0.999。所有相关物质的检出限和定量限分别为0.01%和0.05%。所有杂质的回收率均在85% ~ 105%之间。采用高效液相色谱-紫外分光光度法(HPLC-UV)对VPS注射液在RRT为1.23和1.25的条件下洗脱的未知杂质进行了鉴定和评价。此外,利用FTIR和LC-HRMS进行了聚合谱和二级结构分析研究,将VPS制剂样品与不同稀释剂的参考上市药物(RLD) (Vasostrict)样品进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
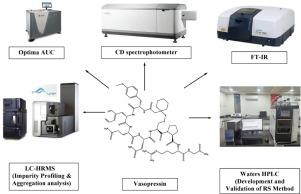
Development and validation of a novel reverse-phase high-performance liquid chromatography (RP-HPLC) method for the quantification of related substances of vasopressin injection: A characterization and comparative analysis of vasopressin formulation samples against vasostrict (RLD) in various diluents
In the present study, we developed and validated a sensitive, accurate, robust and simple RP-HPLC method for quantification of related substances of vasopressin (VPS) injection. The development phase prioritized the optimization of wavelength, mobile phase composition and column selection to enhance separation and sensitivity for the analytical evaluation of VPS injection. By considering peak symmetry, resolution and retention time, chromatographic conditions of VPS injection were established. The YMC PACK ODS AM (100 × 4.6) mm, 3 μm was employed in the study. Mobile phase A was prepared with 0.113 M NaH2PO4.H2O (pH 3.0) while mobile phase B was a 50:50 (v/v) ratio of acetonitrile and water, pumped through a flow rate of 1.0 mL/min. VPS injection was exposed to thermal, photolytic, acid, base and peroxide degradation conditions and these were analysed by the current method. The method was validated following the guidelines set by the International Council for Harmonization guideline (ICH). The linearity studies demonstrating that a correlation coefficient value is more than 0.999 for VPS and its related substances. The detection and quantification limits for all related substances were found to be 0.01% and 0.05%, respectively. All the impurities exhibited consistent recoveries ranging from 85 to 105%. The unknown impurities were eluting at RRT 1.23 and RRT 1.25 in the RS methodology (HPLC-UV) of VPS injection are identified and assessed the impurity levels. Furthermore, aggregate profiles and secondary structure analysis studies were carried out using FTIR and LC-HRMS, comparing the VPS formulation samples with the reference listed drug (RLD) (Vasostrict) samples across various diluents.
Dans la présente étude, nous avons développé et validé une méthode RP-HPLC sensible, précise, robuste et simple pour la quantification des substances apparentées à l’injection de vasopressine (VPS). La phase de développement a donné la priorité à l’optimisation de la longueur d’onde, de la composition de la phase mobile et de la sélection de la colonne pour améliorer la séparation et la sensibilité pour l’évaluation analytique de l’injection de VPS. En prenant en compte la symétrie des pics, la résolution et le temps de rétention, les conditions chromatographiques de l’injection de VPS ont été établies. Le YMC PACK ODS AM (100 × 4,6) mm, 3 μm a été utilisé dans l’étude. La phase mobile A a été préparée avec 0,113 M NaH2PO4.H2O (pH 3,0) tandis que la phase mobile B était un rapport 50:50 (v/v) d’acétonitrile et d’eau, pompé à un débit de 1,0 ml/min. L’injection de VPS a été exposée à des conditions de dégradation thermique, photolytique, acide, basique et peroxyde et celles-ci ont été analysées par la méthode actuelle. La méthode a été validée conformément aux directives établies par le Conseil international d’harmonisation (ICH). Les études de linéarité démontrent qu’une valeur de coefficient de corrélation est supérieure à 0,999 pour le VPS et ses substances apparentées. Les limites de détection et de quantification pour toutes les substances apparentées se sont avérées être respectivement de 0,01 % et 0,05 %. Toutes les impuretés ont montré des récupérations constantes allant de 85 à 105 %. Les impuretés inconnues éluées à RRT 1,23 et RRT 1,25 dans la méthodologie RS (HPLC-UV) de l’injection de VPS sont identifiées et les niveaux d’impuretés ont été évalués. De plus, des profils agrégés et des études d’analyse de structure secondaire ont été réalisés à l’aide de FTIR et de LC-HRMS, en comparant les échantillons de formulation de VPS avec les échantillons de médicament de référence (RLD) (Vasostrict) à travers divers diluants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Annales pharmaceutiques francaises
PHARMACOLOGY & PHARMACY-
CiteScore
1.70
自引率
7.70%
发文量
98
期刊介绍:
This journal proposes a scientific information validated and indexed to be informed about the last research works in all the domains interesting the pharmacy. The original works, general reviews, the focusing, the brief notes, subjected by the best academics and the professionals, propose a synthetic approach of the last progress accomplished in the concerned sectors. The thematic Sessions and the – life of the Academy – resume the communications which, presented in front of the national Academy of pharmacy, are in the heart of the current events.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


