选择性铜- n -杂环碳催化芳基硫鎓盐的C(sp2) -C (sp)交偶联
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究证明了Cu-NHC (NHC = n -杂环碳)通过噻唑-2-乙基配体催化芳基硫鎓盐的炔基化,实现了具有广泛底物相容性和官能团耐受性的无pd Sonogashira偶联。后期的药物烷基化和罕见的烷基化C-H功能化/开环途径被启用。噻唑-2-吡啶具有“半伞”形状的几何结构,比传统的咪唑-2-吡啶具有更好的催化性能,强调了其独特的配体功效。Cu-NHC催化使芳基硫铵盐在温和条件下作为多种交叉偶联的通用亲电试剂使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
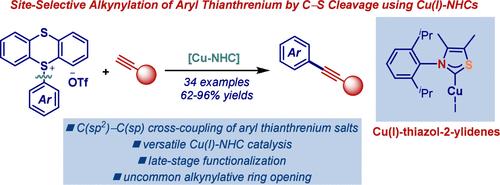
Site-Selective Copper–N-Heterocyclic Carbene-Catalyzed C(sp2)–C(sp) Cross-Coupling of Aryl Thianthrenium Salts
This work demonstrates Cu–NHC (NHC = N-heterocyclic carbene) catalyzed alkynylation of aryl thianthrenium salts via thiazol-2-ylidene ligands, achieving a Pd-free Sonogashira coupling with broad substrate compatibility and functional group tolerance. Late-stage pharmaceutical alkynylation and rare alkynylative C–H functionalization/ring-opening pathways are enabled. Thiazol-2-ylidenes, featuring a “half-umbrella”-shaped geometry, exhibit superior catalytic performance over traditional imidazol-2-ylidenes, underscoring their unique ligand efficacy. Cu–NHC catalysis enables the use of aryl thianthrenium salts as versatile electrophiles for diverse cross-couplings under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


