金属催化的酰基化合物与羟胺衍生物的可调反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种可调的金属酰基亚硝基与酰类化合物的反应,用于合成酰胺类化合物和α,α-二羰基磷酰类化合物。该反应具有条件温和、反应效率好、反应普遍性广的特点。特别是,所得到的酰胺可以在结构上加工成潜在的有用的支架。机理研究表明,漆膜酰胺的形成是由亚硝基铁与硫酰化物的亲核取代反应引起的。人们认为这个反应代表了铁催化亚硝基转移制备酰胺的一个初步例子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
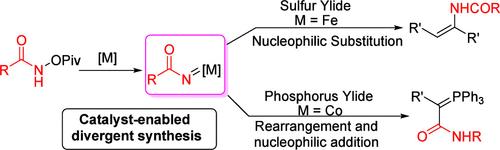
A Metal-Catalyzed Tunable Reaction of Ylides with Hydroxylamine Derivatives
In this work, a tunable reaction of metal acyl nitrene with ylides is reported for the divergent synthesis of enamides and α,α-dicarbonyl phosphorus ylides. The reaction is featured with mild conditions, good reaction efficiency, and broad reaction generality. In particular, the resulting enamides could be structurally elaborated into potential useful scaffolds. Mechanism investigation suggests that the formation of enamides was ascribed to the nucleophilic substitution of ferric nitrene with sulfur ylides. It is believed that this reaction represents an initial example of preparing enamides from iron-catalyzed nitrene transfer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


