羰基与羟基:铑催化羰基基引发2,5-甲烷-1,3-苯并恶氮卓类药物的非对映选择性合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
成功地完成了铑催化n -磺酰基-1,2,3-三唑与2-羟基苯基取代烯酮的化学选择反应。该反应是通过氮杂碳基与烯酮羰基的初始化学选择反应,然后重排和环化发生的。该反应可耐受多种官能团,以单一非对映体的形式高收率合成多种2,5-甲烷-1,3-苯并恶氮卓类化合物。对照实验揭示了势二氢吡咯作为中间体的形成,并有助于提出合理的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
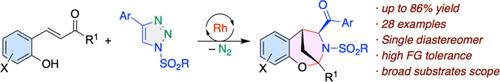
Carbonyl vs Hydroxy: Rhodium catalyzed carbonyl ylide triggered diastereoselective synthesis of 2,5-methano-1,3-benzoxazepines
A general and efficient rhodium catalyzed chemoselective reaction of N-sulfonyl-1,2,3-triazoles with 2-hydroxyphenyl substituted enone has been successfully accomplished. The reaction occurs through the initial chemoselective reaction of azavinyl carbenes to the carbonyl group of enone followed by rearrangement and cyclization. The reaction tolerated various functional groups and allowed the synthesis of various 2,5-methano-1,3-benzoxazepines in high yield as single diastereomer. Control experiments revealed the formation of potential dihydropyrrole as an intermediate and aided in proposing the plausible mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


