在黑暗中揭示胺在原子转移自由基聚合中的作用
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
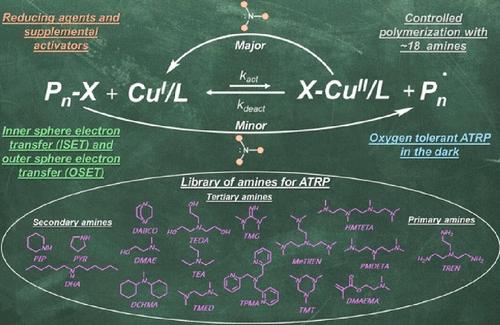
多齿胺在原子转移自由基聚合(ATRP)中被广泛用作cu催化剂的配体,在光化学诱导聚合中被用作电子给体。然而,在黑暗中,胺在ATRP中作用的机制方面仍然难以捉摸。本文研究了25种胺类化合物与Br-CuII /L配合物和/或与烷基溴(R-Br)的构效关系和相关的电子转移反应。胺通过外球电子转移(OSET)机制作为Br-CuII /L配合物的电子供体和还原剂,使CuI/L活化剂缓慢但连续地生成,并诱导可控的ATRP。然而,重氮双环(5.4.0)十一-7-烯(DBU)和1,1,3,3-四甲基胍(TMG)两种胺对Br-CuII /L的还原速度更快,表明存在内球电子转移(ISET)过程。ATRP从初始失活剂(Br-CuII /L)物种开始,在黑暗中进行,存在过量的叔胺,如三[2-(二甲氨基)乙基]胺(Me6TREN), 1,4-重氮双环[2.2.2]辛烷(DABCO)和TMG,在室温下进行,并获得低分散度的聚合物(Đ≤1.15)。利用具有更强还原电位的三酸铜配合物(CuII/L+2, - (OTf)2),在室温下与几种廉价的仲叔胺(包括三乙胺(TEA)和二甲基乙醇胺(DMAE))进行ATRP。有趣的是,多齿胺在高温(60°C)下也可以作为R-Br的直接活化剂。在所有情况下,链都是由R-Br引发的,而不是由胺自由基阳离子作为电子转移的副产物引发的。胺类也使ATRP能够在存在残余空气的烧瓶中具有较大的顶空,使其成为实际应用中坚固且易于使用的还原剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the Roles of Amines in Atom Transfer Radical Polymerization in the Dark
Multidentate amines have been widely used as ligands (L) for Cu-catalysts in atom transfer radical polymerization (ATRP) and as electron donors in photochemically induced polymerizations. However, mechanistic aspects of the role of amines in ATRP in the dark have remained elusive. Herein, the structure–activity relationship and the related electron transfer reactions with Br–CuII/L complexes and/or with alkyl bromides (R-Br) were investigated for 25 amines. Amines function as electron donors and reducing agents for Br–CuII/L complexes via an outer sphere electron transfer (OSET) mechanism, enabling slow but continuous generation of CuI/L activators and inducing controlled ATRP. However, two amines, diazabicyclo(5.4.0)undec-7-ene (DBU) and 1,1,3,3-tetramethylguanidine (TMG), reduced Br–CuII/L faster, suggesting an inner sphere electron transfer (ISET) process. ATRP, starting with initial deactivators (Br–CuII/L) species, proceeded in the dark in the presence of an excess of tertiary amines, such as tris[2-(dimethylamino)ethyl]amine (Me6TREN), 1,4-diazabicyclo[2.2.2]octane (DABCO), and TMG at room temperature and afforded polymers with low dispersities (Đ ≤ 1.15). With copper(II) triflate complex (CuII/L+2, –(OTf)2), which has a more positive reduction potential, ATRP proceeded at room temperature with several inexpensive secondary and tertiary amines including triethylamine (TEA) and dimethylethanolamine (DMAE). Interestingly, multidentate amines also served as direct R-Br activators at elevated temperatures (60 °C). In all cases, chains were initiated with R-Br and not by the amine radical cations as byproducts of electron transfer. Amines also enabled ATRP in the presence of residual air in flasks with a large headspace, underpinning them as a robust and accessible reducing agent for practical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


