用低能光驯服惰性B-H键:近红外光诱导碳硼烷簇-氨基酸偶联的方法
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
碳硼烷簇中惰性B-H键的选择性功能化一直是一个艰巨的挑战。最近的进展已经通过利用紫外线或可见光照射的光氧化还原方法见证了这种反应。然而,高能光源往往遭受能源效率差,有限的衬底范围,不希望的副反应,和低可扩展性。在这里,我们首次使用碳硼烷基电子供体-受体配合物在低能近红外(NIR)光下成功地实现了B-H键功能化。光物理研究和理论模型都表明,在近红外光的照射下,碳硼烷笼基向缺电子光催化剂容易发生单电子转移,生成碳硼烷笼基。后续自由基途径使碳硼烷与氨基酸或寡肽直接偶联,产生多种碳硼烷功能化的氨基酸或寡肽。除了扩大硼簇衍生物的已知化学空间外,我们进一步证明了具有成像和靶向能力的碳硼烷基氨基酸可以作为硼中子捕获治疗中有前途的多功能硼载体。因此,通过近红外光选择性实现碳硼烷的B-H键功能化不仅为硼簇合成化学提供了一种简单实用的策略,而且为下一代含硼生物分子和先进功能材料的开发奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
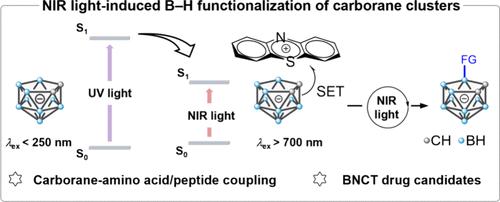
Taming Inert B–H Bond with Low Energy Light: A Near-Infrared Light-Induced Approach to Facile Carborane Cluster-Amino Acid Coupling
The selective functionalization of inert B–H bonds in carborane clusters has been a formidable challenge. Recent advances have witnessed such reactions through photoredox methods utilizing ultraviolet or visible light irradiation. However, high-energy light sources often suffer from poor energy efficiency, a limited substrate scope, undesired side reactions, and low scalability. Here, we present the first successful B–H bond functionalization under low-energy near-infrared (NIR) light using a carborane-based electron donor–acceptor complex. Both photophysical investigations and theoretical modeling reveal a facile single-electron transfer from the carborane cage to the electron-deficient photocatalyst, generating a carborane cage radical under NIR light irradiation. The follow-up radical pathway enables the direct coupling of carboranes with amino acids or oligopeptides, yielding a diverse array of carborane-functionalized amino acids or oligopeptides. Beyond expanding the known chemical space of boron cluster derivatives, we further demonstrate that carborane-based amino acids with imaging and targeting capabilities could serve as promising multifunctional boron carriers for boron neutron capture therapy. Thus, the selective B–H bond functionalization of the carboranes via NIR light not only provides a straightforward and practical strategy in boron cluster synthetic chemistry but also lays the foundation for the development of next-generation boron-containing biomolecules and advanced functional materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


