烯还原酶催化C-C键形成的不对称对映互补合成硫醚
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
酶具有很高的化学、区域和对映选择性,是一种极具吸引力的催化剂。近年来,酶在有机合成中的应用急剧扩大,特别是在手性醇和胺的合成方面,这两个官能团在许多活性药物成分(APIs)中非常重要。事实上,工业界已经描述了许多采用此类化合物的优雅路线。然而,对于手性硫醇和硫醚的合成,同样存在于原料药中,尽管不那么普遍,但只有很少的生物催化合成报道,而且立体控制已被证明具有挑战性。烯还原酶(ERED)具有启动和控制具有化学挑战性的自由基化学反应的能力,我们在此将其应用于从α-溴苯乙酮和原手性乙烯基硫化物合成手性硫醚,而无需光照。根据对 ERED 的选择,可以获得产品的任一对映体。使用含氟底物的 GluER T36A 实现了最高的转化率和选择性,转化率高达 82%,ee 为 99.5%。使用 α-bromoacetophenone 和 α-(methylthio)styrene,反应可以在 100 毫克的规模上进行,产物的分离收率为 46%,ee 为 93%。最后,利用停流光谱法和蛋白质质谱法进行了机理研究,深入了解了酶对分子间反应的偏好。这项工作为合成含硫醚化合物的新路线铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
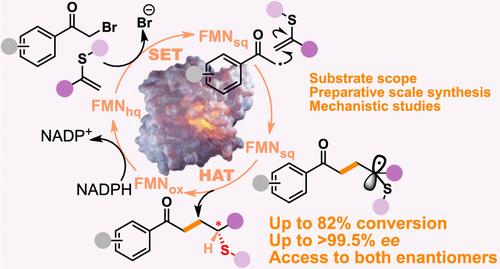
Asymmetric Enantio-complementary Synthesis of Thioethers via Ene-Reductase-Catalyzed C–C Bond Formation
Enzymes are attractive catalysts due to their high chemo-, regio-, and enantioselectivity. In recent years, the application of enzymes in organic synthesis has expanded dramatically, especially for the synthesis of chiral alcohols and amines, two very important functional groups found in many active pharmaceutical ingredients (APIs). Indeed, many elegant routes employing such compounds have been described by industry. Yet, for the synthesis of chiral thiols and thioethers, likewise found in APIs albeit less ubiquitous, only very few biocatalytic syntheses have been reported, and stereocontrol has proved challenging. Here, we apply ene-reductases (EREDs), whose ability to initiate and control chemically challenging radical chemistries has recently emerged, to the synthesis of chiral thioethers from α-bromoacetophenones and pro-chiral vinyl sulfides, without requiring light. Depending on the choice of ERED either enantiomer of the product could be accessed. The highest conversion and selectivity were achieved with GluER T36A using fluorinated substrates, reaching up to 82% conversion and >99.5% ee. With α-bromoacetophenone and α-(methylthio)styrene, the reaction could be performed on a 100 mg scale, affording the product in a 46% isolated yield with a 93% ee. Finally, mechanistic studies were carried out using stopped-flow spectroscopy and protein mass spectrometry, providing insight into the preference of the enzyme for the intermolecular reaction. This work paves the way for new routes for the synthesis of thioether-containing compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


