三组分立体选择性C-N键形成烯烃双官能化
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-02
DOI:10.1039/d5qo00160a
引用次数: 0
摘要
胺是药物、农用化学品和生物活性分子中必不可少的官能团,其C(sp3) -N键在提高生物活性和选择性方面起着至关重要的作用。烯烃双官能团化通过在一个双键上一步引入两个不同的官能团,为构建这些键提供了一种强有力的策略。虽然双组分烯烃双官能化已经得到了广泛的研究,但由于在控制区域选择性、立体选择性和竞争性副反应方面存在挑战,一般的三组分胺合成策略仍然不发达。最近的进展通过过渡金属催化、无导向基方法和基于自由基的机制解决了这些限制,使从现成的起始材料立体选择性合成胺成为可能。本文综述了三组分立体选择性C-N键烯烃双官能化的新策略,重点介绍了机制创新及其对合成有机化学的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
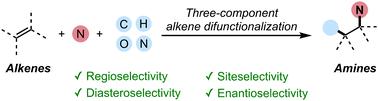
Three-component, stereoselective C–N bond forming alkene difunctionalization
Amines are essential functional groups in pharmaceuticals, agrochemicals, and bioactive molecules, with C(sp3)–N bonds playing a crucial role in enhancing biological activity and selectivity. Alkene difunctionalization offers a powerful strategy for constructing these bonds by introducing two distinct functional groups across a double bond in a single step. While two-component alkene difunctionalization has been widely studied, general three-component strategies for amine synthesis remain underdeveloped due to challenges in controlling regioselectivity, stereoselectivity, and competing side reactions. Recent advancements have addressed these limitations through transition-metal catalysis, directing-group-free methodologies, and radical-based mechanisms, enabling stereoselective synthesis of amines from readily available starting materials. This review discusses emerging strategies in three-component, stereoselective C–N bond-forming alkene difunctionalization, emphasizing mechanistic innovations and their impact on synthetic organic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


