(+)-恩替卡韦的高效全合成
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00329f
引用次数: 0
摘要
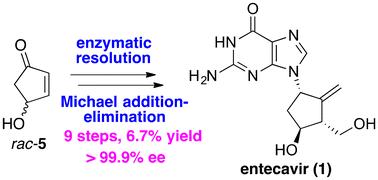
以市售的4-羟基环戊烯-2-烯酮(rac-5)为原料,通过9步完成了恩替卡韦的高效不对称全合成,这是迄今为止该分子最短的不对称全合成。关键反应包括脂肪酶介导的高效动力学分解反应和极具挑战性的立体控制铜催化的Michael加减反应。这项工作为开发生产恩替卡韦的新步骤-经济和具有成本效益的工艺奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An efficient total synthesis of (+)-entecavir†
An efficient asymmetric total synthesis of entecavir was accomplished in 9 steps from the commercially available 4-hydroxycyclopent-2-enone (rac-), representing, to date, the shortest asymmetric total synthesis of this molecule. Key reactions include a lipase-mediated highly efficient kinetic resolution reaction and a highly challenging stereocontrolled copper-catalyzed Michael addition–elimination reaction. This work constitutes a robust basis for the development of a new step-economic and cost-effective process for the production of entecavir.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


