使用Box Behnken设计的塞来昔布负载纳米结构脂质载体的配方,其表征和抗癌评价。
IF 1.1
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
目的:本研究的主要目的是通过肠外途径通过纳米结构脂质载体给药塞来昔布,有效地治疗肺癌,同时克服溶解度、生物利用度、非选择性和负面影响等挑战。方法:采用熔融乳化超声法制备塞来昔布纳米脂质载体,并采用Box-Behnken设计优化。考察了塞来昔布纳米脂质载体的粒径、包封率、zeta电位、体外释放度、细胞毒性、稳定性等指标。结果:优化后的塞来昔布纳米脂质载体包封率为91.69±4.9%,粒径为132.1±6.8 nm,多分散指数为0.41±0.06,zeta电位为-39.1±3.0 mV。值得注意的是,塞来昔布纳米结构脂质载体在pH为6.8的磷酸盐缓冲液中比pH为7.4的磷酸盐缓冲液表现出更好的和可控的塞来昔布释放,揭示了纳米结构脂质载体的肿瘤靶向潜力。此外,塞来昔布从纳米结构脂质载体的释放被控制了48小时,这表明全身毒性的可能性降低了。塞来昔布纳米结构脂质载体对A549细胞的体外细胞毒性是纯塞来昔布的1.5倍,证实了显著的抗肺癌效果。此外,塞来昔布负载的纳米结构脂质载体在低温和环境温度下保持稳定12周。结论:因此,本研究得出结论,纳米结构脂质载体的肠外给药可能是一种无害、有效和新颖的选择,可用于治疗使用塞来昔布的肺癌。本文章由计算机程序翻译,如有差异,请以英文原文为准。
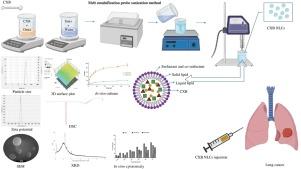
Formulation of repurposed celecoxib-loaded nanostructured lipid carriers using Box-Behnken design, its characterization, and anticancer evaluation
Objectives
The key objective of present research is to effectively treat lung cancer with repurposed celecoxib while overcoming challenges such as solubility, bioavailability, non-selectivity, and negative effects by delivering celecoxib through nanostructured lipid carriers via the parenteral route.
Methods
Celecoxib-laden nanostructured lipid carriers were manufactured by melt-emulsification ultrasonication approach and optimized through Box-Behnken design. The celecoxib nanostructured lipid carriers were examined for particle size, % entrapment efficiency, zeta potential, in vitro release, cytotoxicity, stability, etc.
Results
The optimized celecoxib nanostructured lipid carriers displayed a % entrapment efficiency of 91.69 ± 4.9% and particle size of 132.1 ± 6.8 nm with a polydispersity index of 0.41 ± 0.06, and a zeta potential of −39.1 ± 3.0 mV. Notably, celecoxib nanostructured lipid carriers exhibited better and controlled celecoxib release at phosphate buffer solution pH 6.8 than pH 7.4, revealing the tumor-targeting potential of nanostructured lipid carriers. Also, the release of celecoxib from nanostructured lipid carriers was controlled for 48 h, indicating reduced chances of systemic toxicity. The in vitro cytotoxicity against A549 cells of celecoxib nanostructured lipid carriers was 1.5-fold greater than that of pure celecoxib, confirming significant anti-lung cancer effectiveness. Further, the celecoxib-loaded nanostructured lipid carriers remained stable for twelve weeks at cold and ambient temperatures.
Conclusion
Thus, the given research concludes that parenteral administration of nanostructured lipid carriers could be a harmless, efficient, and novel choice to treat lung cancer using repurposed celecoxib.
Objectifs
L’objectif principal de la recherche actuelle est de traiter efficacement le cancer du poumon avec du célécoxib réutilisé tout en surmontant les défis tels que la solubilité, la biodisponibilité, la non-sélectivité et les effets négatifs en administrant du célécoxib via des transporteurs lipidiques nanostructurés par voie parentérale.
Méthodes
Les transporteurs lipidiques nanostructurés chargés de célécoxib ont été fabriqués par une approche d’émulsification par fusion par ultrasons et optimisés par la conception Box-Behnken. Les transporteurs lipidiques nanostructurés de célécoxib ont été examinés pour la taille des particules, le pourcentage d’efficacité de piégeage, le potentiel zêta, la libération in vitro, la cytotoxicité, la stabilité, etc.
Résultats
Les transporteurs lipidiques nanostructurés de célécoxib optimisés ont affiché une efficacité de piégeage de 91,69 ± 4,9 % et une taille de particule de 132,1 ± 6,8 nm avec un indice de polydispersité de 0,41 ± 0,06 et un potentiel zêta de −39,1 ± 3,0 mV. Notamment, les transporteurs lipidiques nanostructurés de célécoxib ont montré une libération de célécoxib meilleure et contrôlée à une solution tampon phosphate pH 6,8 qu’à pH 7,4, révélant le potentiel de ciblage tumoral des transporteurs lipidiques nanostructurés. De plus, la libération de célécoxib à partir de transporteurs lipidiques nanostructurés a été contrôlée pendant 48 h, indiquant des risques réduits de toxicité systémique. La cytotoxicité in vitro contre les cellules A549 des transporteurs lipidiques nanostructurés de célécoxib était 1,5 fois supérieure à celle du célécoxib pur, confirmant une efficacité anti-cancer du poumon significative. De plus, les transporteurs lipidiques nanostructurés chargés de célécoxib sont restés stables pendant douze semaines à des températures froides et ambiantes.
Conclusion
Ainsi, la recherche présentée conclut que l’administration parentérale de transporteurs lipidiques nanostructurés pourrait être un choix inoffensif, efficace et novateur pour traiter le cancer du poumon en utilisant du célécoxib réutilisé.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Annales pharmaceutiques francaises
PHARMACOLOGY & PHARMACY-
CiteScore
1.70
自引率
7.70%
发文量
98
期刊介绍:
This journal proposes a scientific information validated and indexed to be informed about the last research works in all the domains interesting the pharmacy. The original works, general reviews, the focusing, the brief notes, subjected by the best academics and the professionals, propose a synthetic approach of the last progress accomplished in the concerned sectors. The thematic Sessions and the – life of the Academy – resume the communications which, presented in front of the national Academy of pharmacy, are in the heart of the current events.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


