手性阴离子相转移催化下n -卤化酰胺对2-苯胺苯乙烯的不对称卤环化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以n -卤化酰胺为卤素源,磷酸铵盐为催化剂,在温和反应条件下制备了具有良好官能团耐受性的4h -3,1-苯并恶嗪衍生物。在反应性和立体选择性不降低的情况下,铵盐催化剂负载可降至1 mol %,循环使用4次。重要的是,该工艺的特点是无柱纯化操作,导致绿色和原子经济的手性苯并恶嗪的制备具有良好的收率和高的对映选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
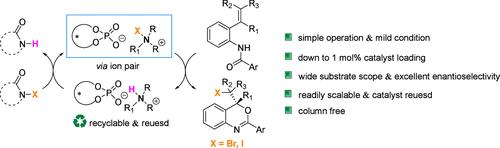
Asymmetric Halocyclization of 2-Anilidostyrenes Using N-Haloamides Enabled by Chiral Anion Phase-Transfer Catalysis
Asymmetric halogenation for efficient construction of various 4H-3,1-benzoxazine derivatives with excellent functional group tolerance using readily available N-haloamides as the halogen source and ammonium phosphate salt catalyst under mild reaction conditions has been developed. The ammonium salt catalyst loading could be reduced to 1 mol % and recycled 4 times without deterioration in reactivity and stereoselectivity. Importantly, this process features a column-purification-free operation, leading to the green and atom-economical preparation of chiral benzoxazines in good to excellent yields and high enantioselectivities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


