溶剂控制4czbnbn催化吲哚羧基酰胺分子内光环化和脱氢光环化合成吲哚喹诺酮类和二氢吲哚喹诺酮类化合物
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00185d
引用次数: 0
摘要
吲哚喹诺酮类和二氢吲哚喹诺酮类是药物化学中急需的基序,但控制合成这两种分子的方法很少。详细介绍了杂环苯胺的溶剂控制可切换光环化和脱氢光环化,以快速合成吲哚喹啉酮和二氢吲哚喹啉酮。以4CzBnBN和DCM/MeOH为催化体系,实现了非对映选择性好、产率高的光环化反应,实现了双氢吲哚喹啉酮类化合物的顺式选择性合成。当溶剂改为DCE/DMSO时,反应途径切换为脱氢光环反应,产生吲哚喹啉酮。该反应的成功与否取决于光催化剂的光物理性质及其与特定溶剂的结合。包括Stern-Volmer猝灭研究、同位素标记实验、Volhard滴定法和DFT计算在内的机理研究表明,光环化反应涉及一个能量传递过程,而脱氢光环化反应同时发生能量传递和电子传递过程。我们的研究不仅为合成吲哚-喹啉酮和二氢吲哚-喹啉酮类药物分子提供了新的策略,而且为氰-诺咔唑基催化剂的催化性能调节提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
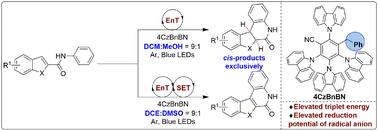
Solvent-controlled 4CzBnBN-catalyzed intramolecular photocyclization and dehydrogenative photocyclization of indole carboxamides for the switchable synthesis of indoloquinolones and dihydroindoloquinolones†
Indoloquinolones and dihydroindoloquinolones are in-demand motifs in medicinal chemistry, yet methods for the controlled synthesis of both molecules are scarce. We detail the solvent-controlled switchable photocyclization and dehydrogenative photocyclization of heterocyclic anilides for the rapid and divergent synthesis of indoloquinolinones and dihydroindoloquinolinones. By using 4CzBnBN and DCM/MeOH as the catalytic system, a photocyclization reaction is achieved with excellent diastereoselectivity and good yields, resulting in the cis-selective synthesis of dihydroindoloquinolinones exclusively. Upon changing the solvent to DCE/DMSO, the reaction pathways switch to dehydrogenative photocyclization that provided indoloquinolinones. The success of this reaction hinges on the photophysical properties of the photocatalyst and its combination with specific solvents. Mechanistic studies including Stern–Volmer quenching studies, isotope labeling experiments, Volhard titration methods and DFT calculations have revealed that an energy transfer process is involved in the photocyclization reaction, while both energy transfer and electron transfer processes occur during the dehydrogenative photocyclization reaction. Our research not only provides a novel strategy for the synthesis of medicinally intriguing molecules of indoloquinolinones and dihydroindoloquinolinones but also offers insights into the modulation of catalytic performance of cyanocarbazole-based catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


