钯通过β-烯基消除z -烯丙醇催化z -烯基化交叉偶联
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00110b
引用次数: 0
摘要
过渡金属催化的z -烯基化交叉偶联已经得到了很好的发展,但这种方法仍然存在局限性。在这里,我们报道了z -烯丙醇和芳基溴之间的z -烯基化交叉偶联。z -烯丙醇的β-烯基消除反应生成了z -烯基钯,并与亲电性芳基溴偶联得到了高收率和立体选择性的z -烯基钯。该工艺具有反应条件温和、底物范围广、官能团耐受性好等特点。此外,它已成功地应用于天然产物和药物分子的后期功能化。在该催化体系中得到了对称和不对称的z -烯基化产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
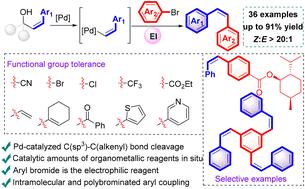
Palladium-catalyzed Z-alkenylative cross-coupling via β-alkenyl elimination of Z-allylic alcohols†
The transition metal-catalyzed Z-alkenylative cross-coupling has been well developed, but limitations remain in this methodology. Herein, we report a Z-alkenylative cross-coupling between Z-allylic alcohols and aryl bromides. The Z-alkenyl palladium species was generated in situ from β-alkenyl elimination of a Z-allylic alcohol, and it underwent coupling with electrophilic aryl bromides to furnish Z-alkenes with high yield and stereoselectivity. This process features mild reaction conditions, wide substrate scope and excellent functional group tolerance. Moreover, it has been successfully applied to the late-stage functionalization of natural products and drug molecules. Symmetric and unsymmetric Z-alkenylation products were obtained in synthetically valuable yields in this catalytic system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


