光催化胺促进烷基系链甲基醚与醛的选择性加氢氯化和sp3 C-O酰化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们提出了一种光氧化还原和烷基胺辅助的方法,用于烷基甲基醚中使用醛的sp3 C-O键的选择性加氢氯化和酰化。该方法利用一系列自由基过程──包括氯自由基加成、氢原子转移、原位亚胺自由基加成和自旋中心移位──来实现炔烃的选择性氢氯化反应和sp3 C-O键的自发裂解。该转化适应广泛的内部炔系醚和醛,为氯烯基酮提供了高效和流线型的途径。该策略仅利用光催化剂、氯化物和丙胺在光照射下,为以前的sp3 C-O裂解方案提供了实用和互补的替代方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
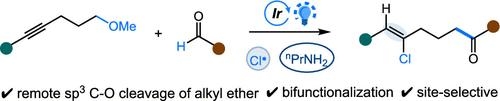
Photocatalytic Amine-Promoted Selective Hydrochlorination and sp3 C–O Acylation of Alkyne-Tethered Methyl Ethers with Aldehydes
We present a photoredox and alkylamine-assisted approach for the selective hydrochlorination and acylation of sp3 C–O bonds in alkynyl methyl ethers using aldehydes. This method leverages a cascade of radical processes─including chlorine radical addition, hydrogen atom transfer, in situ imine radical addition, and spin-center shift─to enable selective hydrochlorination of alkynes and the spontaneous cleavage of sp3 C–O bonds. The transformation accommodates a broad range of internal alkyne-tethered ethers and aldehydes, providing an efficient and streamlined pathway to chloro-alkenyl ketones. Utilizing only a photocatalyst, chloride, and propylamine under light irradiation, this strategy offers a practical and complementary alternative to previous sp3 C–O cleavage protocols.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


