铁催化烯烃区域选择性碳氮化反应合成多取代环丁胺
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-03-22
DOI:10.1039/d5qo00173k
引用次数: 0
摘要
环丁胺是一种多功能合成框架,在药物和天然产品中具有重要的应用。在这里,我们报道了铁催化烯烃的碳氮化反应,使多取代环丁胺的区域选择性合成成为可能。该方法具有底物范围广、官能团耐受性好、合成用途广泛等特点,为构建具有季取代碳中心的多取代环丁胺提供了一种实用的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
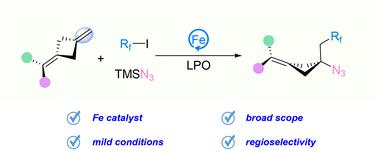
Iron-catalyzed regioselective carboazidation of alkenes for the synthesis of multi-substituted cyclobutylamines†
Cyclobutylamines are versatile synthetic frameworks with significant applications in pharmaceuticals and natural products. Herein, we report an iron-catalyzed carboazidation of alkenes, enabling the regioselective synthesis of multi-substituted cyclobutylamines. This method features a broad substrate scope, excellent functional group tolerance, and versatile synthetic applications, providing a practical approach to constructing multi-substituted cyclobutylamines with a quaternary-substituted carbon center.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


