钯金双金属界面氢化物形成和分解动力学的揭示:光谱和计算相结合的研究
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
负载型钯金双金属纳米颗粒是一类很有前途的催化剂,用于氢化和氧化反应。最近,在纳米颗粒表面和附近的Pd区域的动态重构响应于调节气体(H2和O2)浓度,以控制表面氧化Pd的化学计量学的作用被强调。本文研究了钯氢化物(PdHx)在双金属纳米颗粒表面及其附近的形成和分解机理,PdHx是许多加氢反应中控制钯催化剂活性、选择性和稳定性的关键物质。我们利用调制激发x射线吸收光谱(ME-XAS)直接观察了钯金纳米颗粒表面PdHx形成和分解的时间尺度。密度泛函理论(DFT)计算为不同H组分和Pd亚结构下PdHx形成的稳定性和能量学提供了额外的见解。我们的研究结果揭示了钯系综尺寸、表面结构和氢环境之间复杂的相互作用,决定了PdHx形成的动力学和热力学。通过阐明表面PdHx形成和分解的机理,可以合理设计具有可控氢化钯化学计量的动态催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
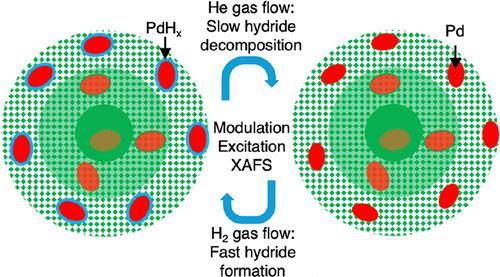
Unraveling the Kinetics of Hydride Formation and Decomposition at Pd–Au Bimetallic Interfaces: A Combined Spectroscopic and Computational Study
Supported Pd–Au bimetallic nanoparticles make up a promising class of catalysts used for hydrogenation and oxidation reactions. Recently, the role of dynamic restructuring of Pd regions at and near the nanoparticle surface in response to modulating gas (H2 and O2) concentrations was highlighted for controlling the surface Pd oxide stoichiometry. Here, we investigate the mechanism of formation and decomposition of Pd hydride (PdHx) at and near the bimetallic nanoparticle surfaces, a key species for controlling the activity, selectivity, and stability of Pd catalysts in many hydrogenation reactions. We employ modulation excitation X-ray absorption spectroscopy (ME-XAS) to directly observe the time scale of PdHx formation and decomposition on the surface of Pd–Au nanoparticles. Density functional theory (DFT) calculations provide additional insights into the stability and energetics of PdHx formation under varying H fractions and Pd substructures. Our results reveal a complex interplay between Pd ensemble size, surface structure, and hydrogen environment in determining the kinetics and thermodynamics of PdHx formation. By elucidating the mechanisms underlying surface PdHx formation and decomposition, the rational design of dynamic catalysts with controlled Pd hydride stoichiometries can become possible.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


