人半乳糖转移酶β3GalT5的结构机制及特异性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
人β1,3-半乳糖转移酶5 (β3GalT5)是参与糖蛋白和糖脂合成聚糖的关键酶,与多种重要的生物学功能有关,特别是与肿瘤的恶性和癌症的进展有关,被认为是开发抗癌药物的一个有前途的靶点。在本研究中,我们测定了β3GalT5与稳定供体类似物udp -2-氟半乳糖或天然供体底物udp -半乳糖(UDP-Gal)和几种聚糖受体配合物在不同反应步骤下的x射线结构。根据实验得到的结构,β3GalT5以类似sn2的机制催化半乳糖从UDP-Gal转移到广谱的聚糖受体;然而,在缺乏聚糖受体的情况下,UDP- gal会缓慢地转化为UDP和另外两种产物,一种是半乳糖,通过类似sn2的机制,以水为受体,另一种是类似氧羰基的产物,可能通过类似sn1的机制。本研究提出的β3GalT5的结构、机制和特异性促进了我们对酶糖基化的理解,并为糖聚糖合成和靶向β3GalT5相关癌症的药物设计提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
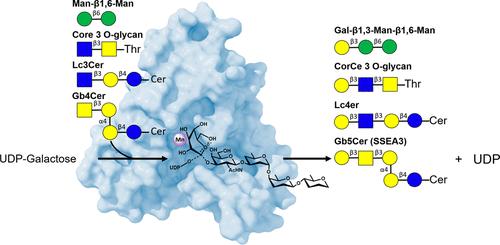
Structure-Based Mechanism and Specificity of Human Galactosyltransferase β3GalT5
Human β1,3-galactosyltransferase 5 (β3GalT5) is a key enzyme involved in the synthesis of glycans on glycoproteins and glycolipids that are associated with various important biological functions, especially tumor malignancy and cancer progression, and has been considered as a promising target for development of anticancer agents. In this study, we determined the X-ray structures of β3GalT5 in complex with the stable donor analogue UDP-2-fluorogalactose or the native donor substrate UDP-galactose (UDP-Gal) and several glycan acceptors at different reaction steps. Based on the structures obtained from our experiments, β3GalT5 catalyzes the transfer of galactose from UDP-Gal to a broad spectrum of glycan acceptors with an SN2-like mechanism; however, in the absence of a glycan acceptor, UDP-Gal is slowly converted to UDP and two other products, one is galactose through an SN2-like mechanism with water as an acceptor and the other is an oxocarbenium-like product, presumably through an SN1-like mechanisms. The structure, mechanism, and specificity of β3GalT5 presented in this study advance our understanding of enzymatic glycosylation and provide valuable insights for application to glycan synthesis and drug design targeting β3GalT5-associated cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


