荧光效应的红外光谱特征源于构象动力学的变化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
全氟烷基和多氟烷基物质因其化学惰性而被广泛应用于社会的合成化合物。这些物质在环境中积累,从那里进入人体,导致癌症等有害影响。PFAS表现出全疏性,这通常导致它们与水相和有机相分离,形成含氟相。然而,这种氟效应的物理性质是未知的。本文通过分析全氟和半氟烷烃在不同溶剂中的红外吸收光谱,揭示了氟效应。我们发现,在含氟和非含氟环境下,C-F拉伸振动区的特定波段表现出选择性行为。在含氟环境中,这些条带显着变宽,而不对称CF3拉伸带强度降低。利用静态密度泛函理论计算和力场分子动力学模拟,我们解读了潜在的分子机制:吸收强度的降低与全氟烷基链的分子间振动耦合有关,而分子碳主链构象变化的加速导致观察到的能带变宽。考虑到所报道的光谱变化对含氟环境的高特异性,在C-F拉伸范围内的波段可以作为含氟相的光谱标记,有利于PFAS聚集的研究。这些知识可以导致PFAS吸收材料的合理设计,其目的是减轻其对环境的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
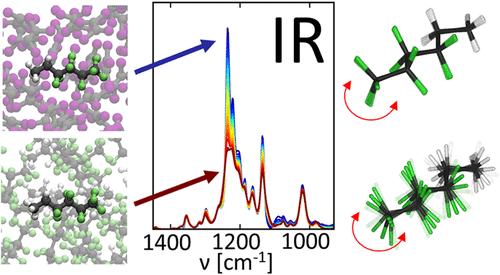
Infrared Spectroscopic Signatures of the Fluorous Effect Arise from a Change of Conformational Dynamics
Per- and polyfluoroalkyl substances (PFAS) are synthetic compounds widely employed in society due to their chemical inertness. These substances accumulate in the environment, from where they enter human bodies, leading to harmful effects like cancer. PFAS exhibit omniphobic properties, which often cause them to separate from both aqueous and organic phases, forming a fluorous phase. Yet, the physical nature of this fluorous effect is unknown. Here, we shed light on the fluorous effect by analyzing the infrared absorption spectra of perfluorinated and semifluorinated alkanes in various solvents. We find that specific bands in the C–F stretching vibrational region exhibit selective behaviors in fluorous and nonfluorous environments. In a fluorous environment, these bands undergo significant broadening, and the asymmetric CF3 stretching bands decrease in intensity. Using static density functional theory calculations and force-field molecular dynamics simulations, we decipher the underlying molecular mechanisms: The decrease in absorption intensities is related to the intermolecular vibrational coupling of the perfluoroalkyl chains, while an acceleration of conformational changes in the carbon backbone of the molecules causes the observed band broadening. Given the high specificity of the reported spectral changes to a fluorous environment, bands in the C–F stretching range can serve as spectroscopic markers for the fluorous phase, facilitating the study of PFAS aggregation. Such knowledge can lead to the rational design of absorber materials for PFAS, which are aimed at mitigating their environmental impact.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


