非局部耦合驱动分子二聚体中的准分子形成:j聚集准分子的情况
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
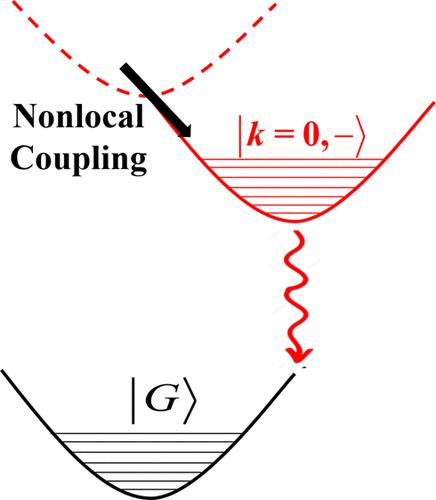
基于 Frenkel-CT-Holstein-Peierls (FCTHP) Hamiltonian,从理论上研究了非局部耦合对分子 H- 和 J-二聚体光物理的影响,目的是确定形成准分子的有利条件。哈密顿方程包括电子耦合以及激子-振动耦合,其中涉及:(1)"快速 "分子内芳香-醌形成模式;(2)"慢速 "分子间(准分子形成)模式,可能涉及简单的拉伸或扭转。非局部耦合源于二聚体复合物沿慢速模式坐标 qs 演变时电子(te)和空穴(th)转移积分的一阶变化,因此用两个参数来描述:ge ≡ dte/dqs 和 gh ≡ dth/dqs,这两个参数在基态几何上进行评估。一般来说,非局部耦合会导致移位和松弛的势能面(PES),这取决于 ge 和 gh 之间的干涉。当非局部耦合参数的符号相反(gegh <0)时,激元在 H 二聚体中更受青睐;当非局部耦合参数的符号相似(gegh >0)时,激元在 J 二聚体中更受青睐。非局部耦合参数之间的干扰也会导致不寻常的 J-和 H-二聚体,在这种情况下,k = 0 和 k = π PES 最小值倒置,从而产生弱发射的 J-二聚体和超发射的 H-二聚体。通过吸收光谱中的振子谱特征可以发现,对于过烯基重色团复合物来说,H-二聚体中的受激体更为有利。了解影响准分子形成的因素是朝着更有效地设计用于设备应用的有机材料迈出的又一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nonlocal Coupling Drives Excimer Formation in Molecular Dimers: The Case for J-Aggregate Excimers
The impact of nonlocal coupling on molecular H- and J-dimer photophysics is studied theoretically, based on a Frenkel-CT-Holstein-Peierls (FCTHP) Hamiltonian, with the goal of identifying favorable conditions for excimer formation. The Hamiltonian includes electronic coupling as well as the exciton-vibrational coupling involving (1) the “fast” progression-forming intramolecular aromatic-quinoidal mode and (2) a “slow” intermolecular (excimer-forming) mode, which can involve simple stretching or twisting. Nonlocal coupling derives from first-order changes in the electron (te) and hole (th) transfer integrals as the dimer complex evolves along the slow-mode coordinate, qs, and is therefore described by two parameters, ge ≡ dte/dqs and gh ≡ dth/dqs, evaluated at the ground-state geometry. Generally, nonlocal coupling leads to shifted and relaxed potential energy surfaces (PESs) that depend on an interference between ge and gh. Excimers are found to be favored in H-dimers when the nonlocal coupling parameters have opposite signs (gegh < 0) and in J-dimers when they have like signs (gegh > 0). The interference between the nonlocal coupling parameters can also result in unusual J- and H-dimers where the k = 0 and k = π PES minima are inverted, resulting in weakly emitting J-dimers and super-emissive H-dimers. It is found that for perylene-based bichromophore complexes, excimers are far more favorable in H-dimers, as identified by vibronic spectral signatures in the absorption spectrum. Understanding the factors which influence excimer formation constitutes an additional step toward the more efficient design of organic materials for device applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


