具有四个不同(杂)芳基取代基的2,3-二氢呋喃的高效级联合成2,3,4,5-四芳基四氢呋喃的立体选择性途径
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
tf2nh介导的1-炔基-2,3-二烷基-2-甲氧基羰基环丙基酮的区域和非对映选择性骨架变态反应容易产生2,3,4,5-四芳基-2,3-二氢呋喃。酯基的可控还原和去除精确地提供了四种可能的四氢呋喃(THF)非对映体,具有四种不同的(杂)芳基取代基。此外,氘标记结果显示,在无buok的条件下,在buok促进的脱碳过程中,甲酰基C-H键发生了前所未有的氢转移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
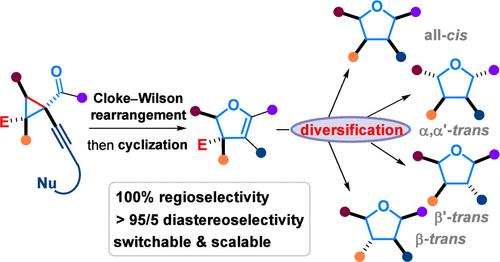
Stereoselective Route to 2,3,4,5-Tetraaryltetrahydrofurans via Efficient Cascade Synthesis of 2,3-Dihydrofurans with Four Different (Hetero)Aryl Substituents
Tf2NH-mediated regio- and diastereoselective skeletal metamorphosis of 1-alkynyl-2,3-diaryl-2-methoxycarbonylcyclopropyl ketones readily gave 2,3,4,5-tetraaryl-2,3-dihydrofurans. Controllable reduction and removal of the ester group then precisely afforded four types of the eight possible tetrahydrofuran (THF) diastereomers with four different (hetero)aryl substituents. Further, deuterium-labeling results revealed an unprecedented hydrogen transfer from the formyl C–H bond in tBuOK-promoted decarbonylation under tBuOH-free conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


